Is the Cause of Some Hospital-Acquired Pressure Ulcers Overlooked?
Hospitals that overlook the role of certain medical devices in causing pressure ulcers are missing an opportunity to prevent as many as one-third of all hospital-acquired pressure ulcers. Patients who develop pressure ulcers are at increased risk of needing longer hospitals stays and of requiring readmission after discharge. They also face a higher risk of dying while in the hospital or after discharge than those patients who do not develop pressure ulcers.
Hospitals must also contend with lost Medicare reimbursement if a Medicare patient develops a preventable pressure ulcer during a hospital stay. As of 2008, the Medicare program stopped paying for the expenses associated with treating advanced hospital-acquired pressure ulcers affecting Medicare beneficiaries. Other third-party payers have adopted the Medicare program's stance of no longer paying for the care associated with these preventable hospital-acquired conditions.
The mere presence of some devices, particularly devices made of rigid material in contact with the patient's skin, puts patients at risk for developing pressure ulcers (Black et al. "Medical Device"). Patient safety events reported to ECRI Institute PSO indicate that patients are developing hospital-acquired pressure ulcers from medical devices and supplies, such as face masks, tubing, and neck collars.
Missed Opportunities for Improvement
Event reports for pressure ulcers, including those that are either hospital acquired or present on admission, represent about 2.5%, or 10,000, of the 400,000 events reported to ECRI Institute PSO. The percentage of reports for pressure ulcers may be higher because some reports of pressure ulcers are sometimes noted in other event report forms, such as those for device-related events.
Given that many pressure ulcer events reported to ECRI Institute PSO occurred after 2008, when the Medicare program eliminated payments for treating advanced hospital-acquired pressure ulcers, there are still opportunities for hospitals to adopt practices shown to be effective in reducing the occurrence of pressure ulcers and, consequently, patient harm.
Organizations that adopt comprehensive measures to prevent pressure ulcers also reduce the risk of claims and lawsuits alleging improper care of patients who develop pressure ulcers. Plaintiffs' attorneys know that cases involving pressure ulcers have a powerful emotional impact on jury members, making defending these cases difficult.
An untapped area for pressure ulcer prevention may be device-related pressure ulcers. By changing some of their approaches for caring for patients with medical devices, organizations could potentially reduce the occurrence of a large percentage of hospital-acquired pressure ulcers.
Indeed, Minnesota, which requires hospitals to report serious adverse health events, found that 35% of all reported pressure ulcers were related to medical devices (Minnesota Department of Health). Given that these are hospital-reported events, the pressure ulcers most likely are hospital-acquired pressure ulcers. Other studies have reached similar conclusions, finding that medical devices contribute up to 35% of all hospital-acquired pressure ulcers (Black et al. "Medical Device").
Senior-Level Support for Change
For pressure ulcer prevention programs—including those targeting medical devices—to be effective, they must have high-level administrative support, as well as support of the staff and managers of units asked to implement the programs (Berlowitz et al.). Senior hospital leaders can foster a pressure ulcer prevention program's success by providing the necessary resources and support to achieve the following:
- Create a system-wide culture of change and commitment to pressure ulcer reduction
- Identify gaps in pressure ulcer prevention current practices
- Assign ownership for pressure ulcer prevention initiatives
- Commit necessary resources for pressure ulcer prevention initiatives
- Educate staff about pressure ulcer prevention on a regular basis
- Enable staff to make pressure ulcer prevention part of their work routines
- Celebrate improvements in reducing the incidence of hospital-acquired pressure ulcers
This issue of the national
PSO Navigator reviews events of pressure ulcers caused by medical devices to identify common findings. The analysis excludes beds, mattresses, and wheelchairs, which can contribute to skin breakdown if patients are bedridden, chairbound, or unable to position themselves. Based on the findings, recommendations to prevent pressure ulcers caused by medical devices are provided in the discussion Lessons Learned. For a summary of these recommendations, refer to "Strategies to Prevent Pressure Ulcers from Medical Devices."
Medical Devices Increase Pressure Ulcer Risk
In 2006, an estimated 503,000 hospital stays for patients over age 18 had a diagnosis of pressure ulcer, according to the Healthcare Cost and Utilization Project (Russo et al.). The data does not indicate the percentage of pressure ulcers that are hospital acquired, although within the Medicare population, an estimated 5% of hospitalized patients develop at least one hospital-acquired pressure ulcer during their stay (Lyder et al.).
Once the circumstances are in place for a pressure ulcer to develop (i.e., pressure against the skin with impeded subcutaneous tissue perfusion), a pressure ulcer can start forming within as little as two hours (Lyder and Ayello). If a medical device is present, the patient is two to four times as likely to develop a pressure ulcer (Black et al. "Medical Device"). These are devices that come in contact with the patient's skin. Examples are listed in "Medical Devices Associated with Pressure Ulcers."
To better understand the circumstances leading to pressure ulcers from medical devices, ECRI Institute PSO conducted a focused review of event reports for pressure ulcers submitted between January 1, 2012, and January 1, 2014. During the two-year period, ECRI Institute PSO received 68,700 event reports using the specific form for pressure ulcers. With a keyword search strategy to identify pressure ulcers caused by medical devices, we retrieved 385 events.
Of the 385 events of pressure ulcers from medical devices, we limited our review to the
123 (32% of the total) that indicated that the event resulted in patient harm. Generally, the reports with higher harm scores provide more detailed narratives for analyzing the events, although we do randomly review events with lower harm scores for additional findings. ECRI Institute PSO's enhanced reporting system uses an index for categorizing errors by nine levels of harm—A through I. This index was originally developed by the National Coordinating Council for Medication Error Reporting and Prevention for reviewing medication errors. For the 123 events, the harm score provided was either E (results in temporary harm and requires intervention) or F (results in temporary harm and requires initial or prolonged hospitalization).
Devices Associated with Pressure Ulcers
Event reports submitted to ECRI Institute PSO indicated a range of devices that can contribute to pressure ulcers. The devices included oxygen therapy devices fitted tightly against the patient's skin; rigid devices, such as braces and cervical collars, that can rub against the skin; various types of tubing resting on and rubbing the patient's skin; and bite blocks, as in the following examples:
An older patient developed a pressure ulcer on his back after wearing a back brace for 24 hours a day.
The patient has a Philly [Philadelphia] collar that was placed in the emergency department. The patient later presented with a pressure ulcer on
the cheek.
The patient was on a ventilator and had a bite block in her mouth because she was biting down on the endotracheal tube. I was changing the endotracheal ties and saw a pressure ulcer on her lower lip from the bite block.
The nurse discovered a pressure ulcer on the patient's nose. The BiPAP [biphasic positive airway pressure] mask was on very tight to maintain a seal because the patient also had a NG [nasogastric] tube in place.
Medical devices, like those reported in these events, cause wounds to form because they create pressure against the patient's skin. A wound can occur if the tissue compression from the medical device remains unrelieved on the area of the body where the device is in contact with skin or mucosal membranes (Murray et al.). Additionally, the presence of the device may cause moisture to develop between the device and skin. Moisture can make the skin more fragile and susceptible to wounds (Black et al. "Use").
Sometimes the devices are secured in place with tape or straps, which, in addition to irritating the skin, can create more pressure from the device and hinder caregivers from inspecting under the device to evaluate the patient's skin for any changes, as in the following event:
The baby had skin breakdown under a facial dressing. The nurse had placed a dressing on the baby's top lip to protect the skin from breakdown from the nasal CPAP [continuous positive airway pressure] mask. The ulcer was discovered when the dressing and nasal CPAP mask were removed to suction the baby.
Improperly positioned devices can also cause a pressure ulcer to develop, as in the following events:
The patient developed a pressure ulcer from the Foley catheter tubing resting against the upper thigh. The patient has delicate skin from edema.
The plastic disc [flange] that holds the feeding tube is too close to the skin. Today I looked under the plastic disc and noted [skin] erosion from the pressure of the plastic.
Even if a device is initially fitted to the patient properly, subsequent swelling can cause a device, such as a brace or cast, to act like a tourniquet and aggravate the skin (Black et al. "Medical Device").
Medical-Device-Related Pressure Ulcers Identified in Later Stages
In reports submitted to ECRI Institute PSO, we found that wounds from medical devices are sometimes undetected because they can be hidden underneath a device like an oxygen mask, tracheostomy tube, or cervical collar. Left undetected, the wound can progress to a later stage.
Most organizations rely on a staging system developed by the National Pressure Ulcer Advisory Panel (NPUAP) and the European Pressure Ulcer Advisory Panel (EPUAP) to determine the severity of a wound. The staging system, ranging from stage I (skin appears reddened) through stage IV (pressure ulcer is deep, reaching into bone and muscle), is used to describe the amount of tissue loss within the wound and to set pressure ulcers apart from other wounds that may appear. The classification system also includes categories for pressure ulcers that are unstageable (the wound extends to the tissue beneath the skin, but its depth cannot be determined), as well as a suspected deep-tissue injury that is difficult to identify. Unstageable pressure ulcers are treated as either stage III or IV. (EPUAP and NPUAP)
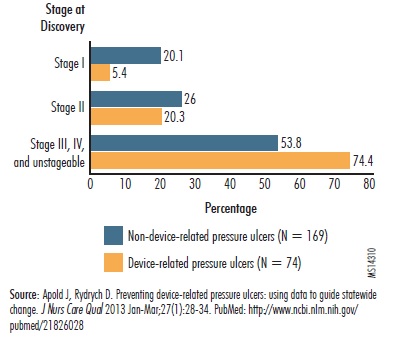
An analysis of Minnesota hospitals' reports of hospital-acquired pressure ulcers found that device-related pressure ulcers were identified at more advanced stages than non-device-related pressure ulcers (Apold and Rydrych). As shown in "Figure 1. Pressure Ulcers Caused by Medical Devices Identified at Later Stages," 74% of pressure ulcers caused by medical devices were found when they were classified as stages III (pressure ulcer extends to tissue beneath the skin) or IV or as unstageable. By contrast, 54% of pressure ulcers caused by factors other than medical devices were classified in these three categories.
Figure 1. Pressure Ulcers Caused by Medical Devices Identified at Later Stages
 |
In addition to the delays in detecting pressure ulcers hidden by medical devices, the events reported to ECRI Institute PSO and the Minnesota analysis offer a few other explanations for why device-related pressure ulcers may develop more quickly to stages III and IV and unstageable wounds. One other possibility is that the devices are located in areas without much fatty tissue (e.g., neck, bridge of nose, openings of the nose, behind the ears) and may progress rapidly. Additionally, the early stage wounds from medical devices are sometimes misidentified as dried-up fluid from the mouth or nose and are undetected until they worsen to a later stage. (Apold and Rydrych)
Medical-Device-Related Pressure Ulcers Frequently Occur near Head
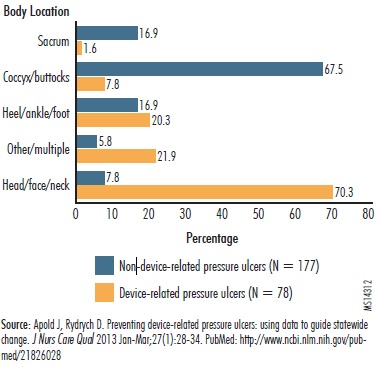
As noted in the reports submitted to ECRI Institute PSO, devices causing pressure ulcers can include neck collars, feeding and tracheostomy tubes, and CPAP and BiPAP masks, all of which are placed around the head, face, and neck. The Minnesota analysis of medical-device-related pressure ulcers found that they are more likely to occur in the area of the head, face, and neck (Apold and Rydrych). In the analysis, 70% of the wounds caused by medical devices occurred on the head, face, and neck, as illustrated in "Figure 2. Pressure Ulcers Caused by Medical Devices Often Located near Patient's Head." By contrast, 68% of pressure ulcers unrelated to a medical device ocurred on the coccyx and buttocks.
Figure 2. Pressure Ulcers Caused by Medical Devices Often Located Near Patient's Head
 |
Devices Impede Skin Assessment
Without regular assessment of the patient's skin under and around a device and under any tape or strap keeping a device in place, the patient is at risk of developing a pressure ulcer that goes unnoticed. Some devices, such as casts and braces, can make it difficult to assess the patient's skin. In the following event, a patient developed pressure ulcers hidden underneath a cervical collar:
The patient was admitted for a brain bleed and placed in a cervical collar. During the morning bed bath, the nurse's aide discovered two pressure ulcers underneath the collar. They are likely stage II and stage III. The pressure points under the collar should be checked.
In another event, a newly admitted patient may have had a previously existing pressure ulcer beneath a dressing holding a feeding tube in place. Upon admission, the skin beneath the dressing was not checked and the wound was undetected:
When the dressing was removed from the feeding tube site, a stage III pressure ulcer was found. There was no documentation of this even though the patient had other documented skin issues. The pressure ulcer developed from the plastic disc holding the feeding tube and causing pressure against the patient's abdomen. The admitting nurse said she had not removed the feeding tube dressing because it was clean, dry, and intact. The importance of removing all dressings and thoroughly checking the patient's skin on admission was reviewed.
In the following event, a pressure ulcer from an endotracheal tube formed beneath the tape holding the tube in place. It was not discovered until the tape was removed:
A small amount of blood was noted on the patient's endotracheal tube taping on her lip. Respiratory therapy was asked to change the taping. Upon removing the tape and repositioning the endotracheal tube, a stage II pressure ulcer was evident.
As part of the skin assessment, any devices that could cause a pressure ulcer may need to be repositioned to relieve pressure on the skin and to evaluate the skin under the device. Some events suggested that staff did not periodically remove or reposition the device, as well as any materials holding the device in place:
The cause of the skin breakdown is a combination of poor perfusion and tension on the endotracheal tube due to positioning. There is no documentation of the tube being repositioned until three days after surgery.
Critically Ill and Young Patients Affected
Patients at risk of developing pressure ulcers from medical devices include those who are critically ill. These patients may require multiple devices, increasing their risk of developing a pressure ulcer from a device. Additionally, they may be sedated and unable to feel pressure from a device or to communicate any discomfort. Critically ill patients may also be too weak to reposition themselves to shift the pressure from the device. (Black et al. "Medical Device")
Events reported to ECRI Institute PSO also highlight the risks to children. In fact, according to some estimates, more than 50% of pressure ulcers in children are due to medical devices (Baharestani and Ratliff). Staff may need to anchor a device to a pediatric patient's skin to reduce the likelihood that the child can pull the device off. As already noted, securing the device with tape can increase the pressure of the device against the child's skin, as well as reduce the detectability of a wound hidden by the tape. Younger patients may not be able to communicate their discomfort from the device pressure and any developing wound (Murray et al.), as in the following event involving an infant:
The infant developed an unstageable pressure ulcer within four hours of placing a catheter to administer medication. The shape of the pressure ulcer is consistent with the shape of one of the catheters. The patient has multiple comorbidities and is at higher risk for skin breakdown.
The report also underscores how quickly a pressure ulcer can develop from a medical device—in this case, within just a few hours.
Multipronged Approach to Pressure Ulcer Prevention
Organizations must adopt multiple strategies to prevent pressure ulcers from medical devices. ECRI Institute PSO recommends that facilities consider a combination of strategies. Some, such as staff education, while important, are considered low-impact strategies and, by themselves, will not prevent every possible pressure ulcer from a medical device. They must be used with other higher-impact strategies, such as standardizing practices to routinely assess the skin of patients who have pressure-causing medical devices. Of course, before any strategies are considered and adopted, the organization should conduct an assessment of current pressure ulcer prevention practices to identify processes that are working and those that need fixing, as well as any gaps in prevention strategies. Refer to "Sample Questions for Gap Analysis of Pressure Ulcer Prevention Practices" for some questions to address during the assessment.
Prevention Starts with Risk Identification
Pressure ulcer prevention starts with an assessment of every patient's pressure ulcer risk. The assessment evaluates the many factors (e.g., mobility, activity, sensory perception, nutrition) that put a patient at risk for pressure ulcer formation during a hospital stay. Based on the assessment findings, care plans can be developed at the time of admission to target individual risk factors for pressure ulcer development and direct preventive measures and treatments.
Some of the commonly used assessment tools (e.g., Braden Scale for Predicting Pressure Sore Risk) do not take into consideration any devices used on the patient that could cause skin breakdown and subsequently progress to a wound (Murray et al.). Nevertheless, the individual or interdisciplinary team performing the patient assessment should include a review of all devices used with the patient to ensure that the care plan addresses management of medical devices that could cause pressure ulcers to form. The assessment should also take into consideration other patient conditions, such as immobility, limited sensation and consciousness, and swelling, that can increase the patient's risk of getting pressure ulcers from medical devices.
Ongoing Patient Assessment
After the initial assessment, skin assessments should be performed on a regular basis, depending on the patient's risk factors (Armstrong et al.). Policies and procedures should dictate how often skin assessments are performed and documented. NPUAP recommends that skin assessments be performed at least daily (NPUAP); however, if the patient has medical devices that increase the risk of pressure ulcers, more frequent and thorough assessments may be necessary. Some have suggested that the skin assessment of patients with pressure-causing medical devices be conducted with each shift (Black et al. "Medical Device"). High-risk patients, such as those with extreme edema, may require even more frequent assessments. The assessment should check the skin for any skin breakdown caused by pressure from medical devices.
Systematic Approach to Skin Assessment
ECRI Institute PSO recommends standardization as a proven strategy to enhance patient safety. An organization should consider a systematic approach to skin assessments so caregivers regularly check the patient for conditions that can cause pressure ulcers to develop as a result of having a medical device on the skin. The organization might even develop a "bundle" of best practices for caregivers to follow for every patient with a pressure-causing medical device. Best practices to consider include the following:
- Lift or move the medical device to examine the skin underneath and other areas vulnerable to pressure ulcers from medical devices (e.g., openings of the nose, inside the mouth). If the patient is obese, examine between any skin folds to ensure the medical device is not hidden from view (Black et al. "Use").
- Apply dressings, as needed, to redistribute pressure and absorb moisture from body areas in contact with a medical device or tubing. Remove and reapply the dressings during skin assessments to check the skin (Black et al. "Use").
- Reposition the medical device at routine intervals (Murray et al.).
- Check the tension of any tape or straps holding a medical device or tube in place to ensure it does not create more pressure from the device against the skin (Murray et al.).
- Check, as appropriate, the fit of devices, such as cervical collars. The Minnesota Hospital Association has developed recommendations to improve the consistency of preventing pressure ulcers from cervical collars and respiratory devices, such as oxygen face masks and endotracheal tubes. Refer to "Online Resources" for information on how to obtain the guidance, as well as other relevant resources on medical-device-related pressure ulcers.
- Examine the patient for any swelling that could aggravate skin breakdown from a medical device. Tape and straps holding a medical device in place could tighten against the device and skin, for example, if the patient experiences swelling. (Black et al. "Use")
- Routinely evaluate the patient's need to continue with a medical device to eliminate unnecessary use of the device. If possible, incorporate prompts in the electronic health record to remind caregivers to reevaluate the need for the medical device.
Staff Education
Root-cause analyses of events of pressure ulcers from medical devices have found that a contributing cause is insufficient staff education about the risks from certain medical devices in aggravating skin breakdown, as well as about the measures to prevent pressure ulcers when a device rests against the patient's skin.
Under state law in Minnesota, facilities must conduct a root-cause analysis of adverse events, such as pressure ulcers, reported to the state's Department of Health. In its 2012 annual report of adverse health events, the state reported that when devices contribute to a pressure ulcer's development, "the root cause of the pressure ulcer is often more closely related to a lack of understanding of risk for skin breakdown with device usage and a breakdown in the implementation of appropriate steps for prevention of the formation or progression of such an ulcer." Further, the report stated that in some cases, "nursing staff may not be familiar enough with the device to know how to inspect under or around it, or policies for when and how such inspections are done may be unclear. In other cases, information related to removal or proper fit of devices may not be effectively communicated from the ordering physician to the care team." (Minnesota Department of Health)
- Organizations should use all available opportunities (e.g., periodic staff education, simulation sessions, unit briefings and huddles) to educate staff about the risks and management of medical devices that can cause pressure ulcers. Education should address the following (Black et al. "Use"; Murray et al.):
- Overview of the organization's policies and procedures for prevention of pressure ulcers from medical devices (e.g., frequency for skin assessments)
- Identification of medical devices that can contribute to skin breakdown and of patients at risk
- Demonstration of best practices for skin assessment and device repositioning
- Demonstration of proper fit for certain devices, such as cervical collars, BiPAP and CPAP masks, and braces
- Resources and staff contacts for additional information
Although pressure ulcers and their prevention are associated with nursing care practices, they are not exclusively a nursing issue. Education should also include physicians (e.g., emergency physicians, anesthesiologists, some surgical subspecialists) who place devices or insert device lines that can cause pressure ulcers.
Interdisciplinary Collaboration
In addition to the patient's bedside nurse, other caregivers may be involved in managing a patient with certain medical devices that can contribute to skin breakdown. For example, a physical or occupational therapist may be called upon to assist with patients who have neck collars, splints, or braces, and a respiratory therapist may be needed for patients requiring respiratory devices, such as ventilators and face masks for CPAP treatment. (Black et al. "Medical Device")
All members of the patient's healthcare team must collaborate to ensure there is a clear understanding of responsibilities. Do team members know who is responsible for routine skin assessments of patients with these devices? Have nursing staff been instructed by therapists involved in a patient's care on how to inspect around any devices applied by the therapist, such as nasal cannulas for respiratory therapy and neck braces for immobilization? Are therapists available to unit nursing staff to assist with any questions about fitting a patient's medical devices or to help make adjustments if a device is uncomfortable for the patient?
Event Reporting
By analyzing pressure ulcer events, organizations are able to identify the factors that contribute to these events and implement strategies to prevent their recurrence. Further, by incorporating standardized event report formats, organizations can explore, at a more granular level, the role of medical devices in causing pressure ulcers. Did a device contribute to the development of the pressure ulcer? If so, what was the device? Are there any device types, in particular, that are problematic for patients?
The Common Formats used by federally certified patient safety organizations for event reports of pressure ulcers provide healthcare organizations the opportunity to answer the following questions about a medical device's role:
- Was the use of a device or appliance involved in the development or advancement of the pressure ulcer?
- What was the type of device or appliance?
- What was the type of tube?
Yet only a minority of the pressure ulcer event reports submitted to ECRI Institute PSO answer these questions, even when a medical device is known to have contributed to the event. ECRI Institute PSO recommends that healthcare organizations use their event reporting systems more consistently to identify whether a medical device contributed to a pressure ulcer event and, if so, to explore the types of devices that are causing the problems. The information collected from event reporting can be used to target solutions to the vexing problem of medical-device-related pressure ulcers and to potentially reduce the number of hospital-acquired pressure ulcers from medical devices.
If the organization has a specific event report form for pressure ulcers, staff should also be instructed to use that form when reporting the development of a pressure ulcer. Important information about medical-device-related pressure ulcers can be overlooked if the data is compiled in a different event report form, such as an event report for medical-device-related incidents. Of course, the organization should emphasize with staff that event reporting is used to improve patient care, not to punish staff for situations that are often the result of system flaws.
If the organization collects information about a particular medical device that repeatedly contributes to patient's skin breakdown and the development of pressure ulcers, the organization should also consider reporting the information to the manufacturer and to the U.S. Food and Drug Administration's (FDA) medical device reporting program (Black et al. "Use"). Refer to "Online Resources" for information about FDA's reporting program.
Product Evaluation
As a preventive strategy, healthcare organizations should assign their product evaluation committees with responsibility for assessing a particular medical device or supply for any risks they present for patients. These risks include skin breakdown and pressure ulcer development. The committee may even want to involve staff who will be using the devices, such as nurses and therapists, for their input on any noticeable device hazards. If the organization intends to purchase the product, the committee should review any directions for use that address skin breakdown and ensure that these instructions are communicated to staff and incorporated into policies and procedures for skin assessment and device management (Murray et al.).
The product evaluation committee members may also want to collect feedback from staff and from event report data to determine whether any pressure-ulcer-related problems have occurred with devices and to investigate whether alternative device management techniques or alternative products are needed.