Consequences of Suboptimal Reprocessing
Every day, healthcare facilities' sterile processing departments handle thousands of reusable surgical instruments and devices—ranging from knife handles and forceps to arthroscopic shavers and fiberoptic endoscopes. The department reprocesses the used instruments so they are ready for subsequent procedures. Suboptimal reprocessing practices can mean that instruments that have gone through the department are returned to the operating room (OR) and other procedure areas—such as cardiac catheterization laboratories and ambulatory settings—with human tissue, bone, or other organic material in or on the treated instruments.
The consequences can be disastrous for patients, staff, clinicians, and the organization. Significantly, patients are at risk of infections from dirty instruments used on them. Even if a soiled instrument is discovered before it is used on a patient, there could be procedure delays while the healthcare team waits for clean instruments, possibly resulting in extended periods of time for patients under anesthesia. Disruptions from these incidents may also lead to other errors. In addition to the risks to patients (as well as the anxiety these events cause them), incidents involving improperly reprocessed instruments can damage an organization's and its providers' reputations, reduce patient satisfaction, result in citations and fines from regulatory bodies, prompt review by accrediting agencies, and lead to lawsuits.
Therefore, healthcare executives must pay close attention to the activities of their sterile processing departments, which have an essential role in the delivery of safe patient care. Even changes in equipment, personnel, and procedures can affect the organization's ability to provide properly reprocessed instruments. If instruments are reprocessed in any other areas of the facility, executives should ensure that consistent procedures are in place throughout the facility.
Reports of Dirty Instruments
ECRI Institute PSO and other event reporting programs, including the U.S. Food and Drug Administration (FDA), have received reports of dirty instruments that were not adequately decontaminated and cleaned before they underwent disinfection or sterilization. Some, but not all, contaminated instruments were detected before they reached patients. If the soiled instruments are used during patient procedures, patients are at risk of postoperative infections, such as surgical site infections and the transmission of potentially deadly infectious agents that cause diseases such as hepatitis C, HIV, and tuberculosis. Organizations must then notify patients of possible exposure to infectious material and monitor the notified patients for infection.
Incidents like these have the attention of state and federal regulators and the Joint Commission. Both the Centers for Medicare and Medicaid Services (CMS) and the Joint Commission are more closely monitoring healthcare facilities' attention to infection control and instrument reprocessing procedures and the facilities' adherence to recommended guidelines and practices. At the state level, state departments of health and others are involved in these issues, partly as a result of federal funding, provided in the American Recovery and Reinvestment Act of 2009, to prevent healthcare-associated infections, including surgical site infections. Reducing preventable surgical site infections is also a goal of CMS's Partnership for Patients.
Joint Commission data suggests that there is still room for improvement. The agency reports that 36% of accredited hospitals surveyed in 2011 were noncompliant with its standards to reduce the risk of infection associated with medical equipment, devices, and supplies. Failure to adhere to the standard, which includes measures to properly decontaminate, clean, disinfect, and sterilize medical equipment, was among the top 10 standards compliance issues for hospitals, ambulatory settings, and office-based surgery practices in 2011 (Joint Commission).
More Complex Instruments: Harder to Clean
Experts agree that there is an increase in the number of cases of improperly cleaned instruments reaching end users. One of the chief culprits is changes in technology. Before the advent of minimally invasive surgery, most instruments used in the OR were made of stainless steel and reprocessed with standardized methods. Today, instruments for procedures ranging from colonoscopies to robotic-assisted laparoscopic surgery are more complex and often have movable parts to disassemble and narrow channels to clean, says Gail Horvath, M.S.N., B.S., RN, CNOR,
CRCST, patient safety analyst at ECRI Institute. See "Tough to Clean" for a list of instrument features that make reprocessing difficult.
Although no one knows the extent of the problem of improperly reprocessed instruments, data from FDA and CMS is noteworthy (Schaefer):
FDA received 80 reports of inadequate reprocessing between January 2007 and May 2010; 28 reports of infection may have occurred from the inadequate reprocessing.
CMS investigated infection control practices at ambulatory surgery centers in 2008 and found that 28% of the facilities had some type of lapse in reprocessing medical equipment.
The number of reported incidents of contaminated reusable instruments reaching end users is the "tip of the iceberg," says one official from the Centers for Disease Control and Prevention (CDC), which has investigated reports of infection outbreaks from dirty instruments. The official spoke at an FDA workshop held in 2011 to identify, discuss, and formulate strategic initiatives and priorities to improve reusable medical device reprocessing (Schaefer).
In addition to the challenges from cleaning more complex instruments, other factors
contributing to the increase in reported incidents include the following:
- Pressure on sterile processing departments to quickly turn around instruments for scheduled procedures due to insufficient instrumentation; staff may even resort to risky shortcuts.
- An inefficient work environment, prone to distractions, in the sterile processing department.
- Lengthy and unclear manufacturer instructions for cleaning instruments.
- Delays in obtaining from a vendor "loaner" instruments that need to be reprocessed before they are made available for surgical cases and other procedures.
- After-hours requests for instruments requiring reprocessing outside of the sterile processing department's normal hours of operation.
- Inadequate training of personnel outside of sterile processing who can accommodate after-hours requests for instruments and equipment.
- Poor communication between OR and sterile processing staff about each department's needs.
- Multiple sterile reprocessing areas without standardization of processes.
Prevention Is Possible
Fortunately, healthcare organizations can adopt measures and practices to prevent incidents of contaminated instruments reaching end users and patients (see "Top 10 Essentials for Effective Instrument Cleaning" for a summary of these strategies). This issue of the
PSO Navigator describes events involving dirty instruments submitted to ECRI Institute PSO and other reporting agencies and provides recommendations to improve reprocessing practices, with a focus on instrument decontamination and the cleaning that occurs before disinfection or sterilization.*
Healthcare leaders can provide much-needed support for these initiatives by:
- Recognizing the important contribution of staff in the reprocessing department to safe patient care and to the organization's daily operation—particularly in keeping the OR, the dominant revenue-generating department, supplied with essential instruments.
- Providing adequate staff, space, and equipment for the sterile processing department to effectively function.
- Allocating resources to ensure that ORs and other procedure areas have sufficient instruments to meet demand, allowing for adequate time for instrument processing.
- Requiring standardization of procedures in all reprocessing areas.
- Supporting education of sterile processing technicians. Some organizations provide for certification of staff, and
New Jersey requires certification for personnel performing sterile processing functions in hospitals and other settings such as doctors' and dentists' offices. - Requiring regular competency assessment of any staff involved in reprocessing.
- Fostering teamwork and collaboration among staff in the OR and sterile processing departments.
* In addition to the review provided by ECRI Institute PSO’s Advisory Council, ECRI Institute PSO acknowledges the input provided by Nancy Chobin, RN, CSPDM, assistant vice president, sterile processing services, Barnabas Health, West Orange, New Jersey.
Breakdowns in Reprocessing Compromise Safety
Healthcare staff and clinicians often falsely perceive that sterilization alone is adequate to ensure instruments are ready for reuse. In fact, device reprocessing is a multistep practice that includes proper cleaning followed by disinfection or sterilization (see "Figure. In-House Reprocessing of Reusable Instruments").** Reprocessing is primarily conducted in the sterile processing department, also known as central supply, central sterile, processing, central processing, and supply processing, among other names. However, not all instrument reprocessing is centralized within the department; for example, many healthcare facilities' endoscopy suites reprocess their own instruments. A breakdown in any of the reprocessing steps can compromise the integrity of the process, leading to an instrument-related contamination risk.
** For this article, the term “reprocessing” refers to in-house practices to prepare reusable instruments and equipment for reuse. The term should not be confused with third-party reprocessing companies that prepare single-use devices for reuse.
FDA requires device manufacturers to provide instructions for reprocessing their devices and to validate these procedures. Hospitals have raised concerns that some instructions are unclear,
extremely complicated, and lengthy. In response, the agency has developed draft guidance to improve manufacturers' reprocessing instructions and is reviewing comments on the draft, which was issued in 2011. See "Reprocessing Guidance" for information on accessing FDA's guidance online.
Figure. In-House Reprocessing of Reusable Instruments
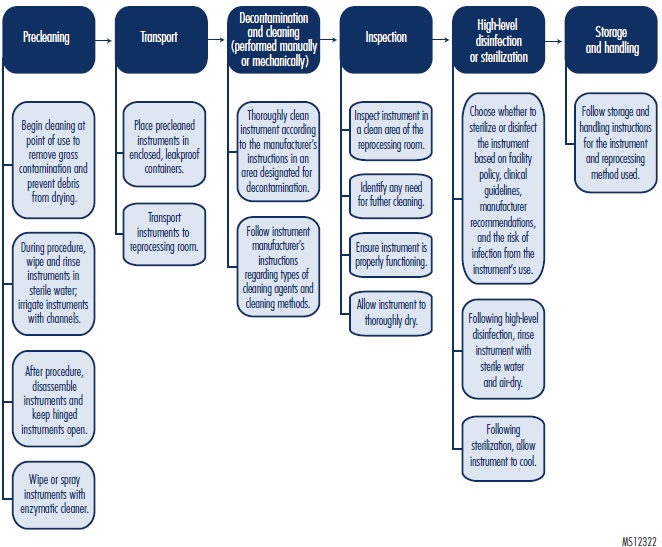 |
Cleaning of the used instrument actually begins at the point of care, says Horvath. For example, in the OR, the scrub person removes any gross contamination, such as blood, tissue, and other obvious debris, from the equipment with sterile water. To begin decontamination (the removal of infectious agents), the instruments are soaked in an enzymatic solution or covered with a spray that initiates decontamination.
The items are then sent to sterile processing in leakproof containers to minimize the risk of any contamination to the environment and personnel. The instruments are more thoroughly decontaminated and cleaned in a specially designated decontamination area of the sterile processing department. Cleaning can be done either manually or with equipment, depending on the manufacturer's recommendations.
After cleaning, the instruments undergo either high-level disinfection or sterilization, depending on the intended use of the instruments. Refer to "ECRI Institute Guidance" for more information about these steps.
Meticulous Cleaning Necessary
As events reported to ECRI Institute PSO and others indicate, failure to meticulously clean and inspect instruments after cleaning can interfere with high-level disinfection and prevent sterilization, increasing the risk of infecting a patient if the instrument is used later during a procedure or contaminating the sterile field if the dirty instrument is brought into the field. Additionally, an instrument can be damaged from prolonged contact with organic material that remains on it.
In one report submitted to ECRI Institute PSO, the OR team had to request two instrument trays until it was provided a third tray that was ready for use. The patient was under anesthesia once the first replacement tray was returned:
Case 1.
Bone and tissue were observed in the instrument tray for joint replacement surgery. The tray was removed, and a new sterile field and replacement instruments were set up in the room. The replacement instrument tray had fluid on several instruments and bone fragments. The second setup was broken down, and a new setup was opened using sterile technique.
In another report, the start of surgery was delayed in order to obtain new instruments to replace those that were contaminated:
Case 2.
When opening the OR supplies for a surgical procedure, we noticed blood on the instrument bin inside the case cart. All the instruments were contaminated, and the supplies in the cart had to be thrown away. The start of surgery was delayed while new supplies were obtained.
In other reports, the contaminated instruments were used on patients; in one case, the physician performing the procedure mistakenly obtained an instrument that had not been reprocessed. In these two cases, the patients were informed of the incidents and the need for monitoring to determine if an infection develops:
Case 3.During surgery to repair a patient's rotator cuff, the surgeon found a foreign substance in the arthroscopy shoulder cannula. The patient required monitoring.
Case 4.A patient had to undergo bronchoscopy after normal working hours. The physician obtained a scope from the pulmonary lab. After the procedure was completed, it was determined that the scope had not been reprocessed from the previous procedure. The patient was notified.
Another report underscores the need to properly clean an instrument when immediate-use steam sterilization is used in the OR. Previously known as "flash sterilization," immediate-use
sterilization is a way to sterilize medical instruments that must be immediately transferred into the sterile field for use. It is recommended only for use in emergency situations and is not to be used for convenience or as a substitute for insufficient inventory.
Case 5.During a surgical procedure, part of an implant was dropped. The implant was flash sterilized so that it could be used, but the proper flash sterilization process was not followed. Inadequate time was allotted for cleaning the implant.
One other report submitted to ECRI Institute PSO, while not about a contaminated instrument, hints at the disrespect sometimes displayed toward sterile processing staff, whose education may be limited to a high school degree or equivalency degree. Lack of respect for sterile processing workers hinders effective communication between that department and the end user and, ultimately, can affect patient safety.
Case 6.The cannulated screw set was stocked by central sterile processing with screws of the wrong length. Interesting that this does not happen when a product rep brings the trays.
Strategies to Improve Reprocessing Practices
ECRI Institute has identified patient cross-contamination from improperly reprocessed flexible endoscopes as one of its top 10 health technology hazards (to access the most recent report, see "ECRI Institute Guidance"). As the reports submitted to ECRI Institute PSO illustrate, similar concerns about improper reprocessing extend to other reusable medical and surgical instruments. These incidents resulted from failure to follow established cleaning guidelines before sterilization and high-level disinfection. Listed below are strategies to improve the reprocessing of instruments, with a focus on instrument cleaning. A discussion of disinfection and sterilization techniques is beyond the scope of this article.
Audit policies and procedures. Effective reprocessing depends on strict adherence to evidence-based standards and recommended practices. Organizations' policies and procedures should be based on national guidelines and standards, such as the Association for the Advancement of Medical Instrumentation's (AAMI)
Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities and the CDC's
Guideline for Disinfection and Sterilization in Healthcare Facilities. These and other guidelines and standards establish an optimal level of practice for settings that reprocess reusable instruments. Organizations should ensure that the recommendations are adapted for all practice settings where reprocessing occurs. See "Reprocessing Guidance" for information on obtaining various national guidelines and standards.
Standardize and simplify. At FDA's 2011 workshop on medical device reprocessing, Linda Condon, M.B.A., RN, CRCST, educator, central sterile processing department at the Johns Hopkins Hospital (Baltimore, Maryland) discussed the challenges of reprocessing a vast number of medical devices from different manufacturers using instructions provided by manufacturers that are often lengthy (some instructions are 100 pages or longer), variable from one device to another, and confusing. The hospital has about 24,000 unique instruments and reprocesses about 40,000 items daily. To simplify and standardize the cleaning and decontamination instructions for department staff, Condon tells ECRI Institute PSO that the department has developed one-page, quick-reference sheets with manual cleaning instructions for about 100 categories of medical devices. Each laminated reference sheet has a standardized layout and uses consistent terminology. For now, printed copies of the reference sheets are available for staff reference, although Condon plans to make the reference sheets available electronically so that when a device is scanned, the appropriate, one-page cleaning instructions are displayed on a computer. "We're trying to apply standardization because every manufacturer's instructions for use are different," says Condon. Additionally, every instrument that is brought to the decontamination area is electronically scanned before cleaning; the computerized information indicates whether the item is sorted into a zone for either manual or mechanical cleaning.
Standardized processes should be adopted in all areas where instruments are reprocessed, including endoscopy suites. The Johns Hopkins Hospital has also applied standardization to its sterilization area with standard operating procedures for its sterilization methods. Additionally, to prevent mix-ups of sterile and nonsterile items, the department uses visual cues, such as color-coded floor markings and carts, to signify sterile and nonsterile zones; orange and red flags hung on carts to identify nonsterile equipment; and clocks and signs placed on sterile carts to indicate the cooling time for sterile items. (Condon)
Allocate resources for sterile processing departments. To function effectively, sterile processing departments require adequate space—particularly as the number of instruments requiring reprocessing increases—and resources, including large sinks for cleaning, brushes of varying sizes for manual cleaning, and magnifying lights so technicians can closely inspect instruments for any remaining debris after they are cleaned. Some facilities, including Hopkins, also pay third-party contractors to maintain a database of manufacturers' instructions for reprocessing and to check on a periodic basis—often quarterly—for any updates to the instructions. Unfortunately, healthcare facility budgets can overlook their sterile processing departments as a vital resource for patient safety given that they are not revenue-generating
operations and are often located in hospital basements, far from the public's and many staff members' view.
Healthcare leaders must also allocate funds to provide a sufficient inventory of instruments so that the sterile processing department and OR can turn around the reprocessed instruments without resorting to shortcuts, depending on immediate-use sterilization, or borrowing instruments from vendors.
Limit immediate-use sterilization. Some OR departments rely heavily on immediate-use sterilization to ensure instruments are available for an upcoming procedure despite recommendations from CDC and others discouraging immediate-use sterilization as an alternative to purchasing new equipment or as a matter of convenience. There are limitations to this sterilization method, such as the lack of methods to monitor, in a timely manner, its effectiveness in sterilizing items. Additionally, as one event reported to ECRI Institute PSO suggests, OR staff, in a hurry to sterilize an item, may fail to adequately perform all the necessary steps for immediate-use sterilization, which includes proper cleaning of the item. "Decontamination and cleaning are just as important with immediate-use sterilization," says ECRI Institute's Horvath. OR staff who use this sterilization process must receive training, she notes. Ideally, however, less than 10% of surgical cases should use immediate-use sterilization, she recommends. Organizations should monitor their immediate-use sterilization rate by establishing a target rate and tracking their success in meeting that rate.
Establish procedures for loaner instruments. Many healthcare facilities rely on vendors to provide "loaner" instruments for specialty procedures, such as orthopedic implant surgery, for which they cannot maintain an inventory of special devices. When these loaner instruments arrive at the healthcare facility, the sterile processing department requires adequate time to reprocess the items. "You can't assume loaner equipment has arrived at the hospital properly decontaminated and cleaned," says Horvath. Sterile processing department workflow may be interrupted if the loaner instruments arrive shortly before a scheduled operation and require immediate attention. To avoid delays, some healthcare facilities establish a designated time frame (e.g., 24 hours before a scheduled case) for the delivery of loaner instruments to the facility and specify that they be accompanied by manufacturers' instructions for reprocessing, says Horvath.
Address ease of device cleaning before purchase. Healthcare facilities might consider involving a representative from their sterile processing departments in instrument purchase decisions in order to evaluate, before its purchase, a reusable device's reprocessing needs and the accompanying manufacturer's instructions. If the device requires reprocessing methods that are outside the department's capabilities, it may be an inappropriate purchase for the organization. Likewise, the representative can evaluate any device that must be disassembled for reprocessing and how easily it can be taken apart. Devices that are difficult to disassemble may also be difficult to clean. Senior leaders at the Johns Hopkins Hospital have also recently given the sterile processing department the authority to refuse to accept any instrument that does not conform to the department's processes. Although most reusable instruments are evaluated by the department before purchase, a few instruments still escape the department's review. "We're a big system, so things get purchased without our assessment," says Condon, noting that "most departments are learning to come to us before a device is purchased."
Inspect instruments after cleaning. Before being sterilized or placed in storage, a decontaminated instrument should be inspected for cleanliness and device function. Otherwise, an item with contamination that is undetected during the inspection process is likely to be returned to clinical use after it is sterilized. The Johns Hopkins Hospital has identified certain difficult-to-clean instruments with lumens and channels that must be carefully evaluated after cleaning. The hospital uses a product to identify any remaining organic material inside these instruments, which include:
- Instrumentation for minimally invasive robotic surgery
- Flexible scopes
- Aneurysm suction catheters
- Cannula for extracting bone marrow specimens
- Arthroscopic shavers, which require a video scope to inspect the channels of the handpiece
Ensure technician competency. AAMI's guidelines on steam sterilization recommend that healthcare facilities require, as a condition of employment, that sterile processing personnel successfully complete a central service certification examination within two years of employment and maintain the certification throughout their employment. "Certification demonstrates that staff have the knowledge and critical-thinking skills to perform their jobs," says Horvath. New Jersey is the only state to require certification of personnel performing sterile processing functions, and at least three other states—New York, Ohio, and Pennsylvania—are considering similar measures. Both AAMI and the New Jersey Department of Health recognize certification programs from the Certification Board for Sterile Processing and Distribution and the International Association of Healthcare Central Service Materiel Management. Certification exams test technicians' knowledge and skills in infection control and sterile processing practices. In addition to certification of their staff, healthcare organizations should require regular competency assessment of any staff involved in reprocessing.
The Johns Hopkins Hospital, which prefers certification of its sterile processing department staff, has established four tiers of job descriptions for its staff and requires certification for the two highest-level positions, says Condon. "We've created a career ladder within the department with higher pay," she says. Additionally, the hospital provides ongoing education for its staff in cleaning and sterilization techniques and conducts ongoing audits of staff performance.
Because it has measures in place to support the proficiency of its sterile processing staff in cleaning and sterilization techniques, the Johns Hopkins Hospital tries to ensure that most instruments are reprocessed by the department rather than in the areas where the instruments are used. "We are looking to centralize as much of the process as we can," says Condon. In those areas, such as endoscopy suites, where some instrument reprocessing is performed in the unit, staff members are specifically trained in reprocessing methods, she says.
Foster collaboration. Sterile processing and OR staff, as well as staff from other procedural units, depend on each other to correctly perform their jobs so that the organization can provide safe patient care. Each department must educate the other on how they can work together. At the Johns Hopkins Hospital, for example, the sterile processing department has developed standard operating procedures for perioperative staff to follow to ensure adequate precleaning of instruments before they are sent from the department for reprocessing.
OR staff can also assist reprocessing staff by keeping used instruments together in sets when they are sent for reprocessing and developing inventory lists for instrument trays that are reassembled in the processing department. At the start of a procedure, OR staff should be careful to choose only the instruments and supplies that are needed and avoid opening the packages of items that should be on hand but may not be needed. Unused items must still be reprocessed if they are taken out of their sterile packaging. One author also recommends that sterile processing staff check daily with perioperative staff to discuss needs for upcoming procedures and that a representative from the department participate in OR staff meetings to foster communication between the two departments (Seavey).
Collaboration will not occur unless the organization supports a culture of respect and dispels the sometimes privately held belief that sterile processing staff are "glorified dish washers" (Swanson)—as reflected in the tone of one of the reports submitted to ECRI Institute PSO. When contaminated instruments are unintentionally provided to staff in the OR or other procedures areas, the errors are not always the fault of individuals in the reprocessing department but are more typically the result of flawed processes. Adopting many of the strategies suggested in this article will help to promote a culture of safety and environment of trust.
Report and analyze events. Organizations can learn from patient safety events by systematically reviewing them to discover the factors that contributed to them and identifying prevention strategies. As with any event reporting program, the facility must foster a culture in which caregivers recognize the importance of reporting events and near misses involving contaminated instruments as part of the organization's overall commitment to safety. Organizations should share the findings of their analyses of the events so staff can understand how system issues—not individual staff members—contributed to the problem and are aware of the measures put in place to prevent similar events.
Organizations should also establish a process to identify any events that should be reported to FDA, CDC, manufacturers, and others. When one Texas hospital identified a cluster of infections occurring with arthroscopic procedures performed over a two-week period, the hospital enlisted the help of FDA and CDC, which identified problems with the manufacturer's reprocessing instructions for its arthroscopic shavers. The findings prompted FDA to issue a safety alert about the risk of tissue remaining within certain arthroscopic shavers, even after cleaning according to the manufacturer's instructions. The alert recommended measures to improve cleaning of the devices. (Tosh et al.; U.S. FDA )
Prompt reporting of improperly reprocessed instruments is also essential to determining whether any patients are at risk from contaminated instruments used during their treatment and to beginning notification of the patients. Healthcare facilities must implement measures to capture identifying information about instruments used on patients in patients' medical records to facilitate patient notification.