Portable/transport ventilators are compact, lightweight devices used to provide breathing support in a variety of applications. Today’s models have longer battery life and fit an increasing range of capabilities into a small package. Many of these devices boast performance approaching that of full-size ventilators.
These capabilities have changed the way these ventilators are being utilized: Instead of being used only during patient transport, portable models are being used as the main ventilator in some organizations and hospital departments. In fact, one of the models in this Evaluation—the Hamilton Medical C2—is marketed as meeting the complex ventilatory needs of the intensive care setting.
But not all applications demand such an extensive set of features. In long-term care, for instance, simplicity and low cost are a plus, and pricey advanced features may go unused.
It is important to know exactly which capabilities you need and to find a product with features to match.
We tested the following products:
We also tested a different type of ventilator, designed specifically for use in mass-casualty situations:
- Allied Healthcare Products MCV100
The MCV100 is a very inexpensive, minimally equipped unit that falls within a category that we call “supplemental mass-casualty” ventilators, since a facility must use it in conjunction with an inventory of more capable models in order to meet the needs of the full range of expected victims following a mass-casualty event. We have therefore evaluated this unit separately from the others. See the discussion of supplemental mass-casualty units, as well as our ratings and test results for the MCV100.
_________________________________________________________
New Rating System
In this Evaluation, we introduce our new five-star rating system. Products we consider suitable for purchase are given a rating of one to five stars. This system replaces our former ratings of Preferred, Acceptable, and Not Recommended. We’ll still use a rating of Unacceptable if we evaluate a product that is completely unsuitable for purchase or use. (No such products are covered in this article.)
_________________________________________________________
The applications for which we rated the products are:
In-hospital transport. Since many critically ill ventilated patients now undergo frequent diagnostic tests, having a portable/transport ventilator available that can mimic the performance of an intensive care ventilator is beneficial. The following features are essential for aiding clinicians in setting the portable ventilator to deliver ventilation similar to an intensive care ventilator: synchronized intermittent mandatory ventilation (SIMV) mode, pressure-control breaths, controls for flow profiles, and a high-pressure oxygen inlet, as well as the ability to specify oxygen concentration. Degree of portability, including weight, is also an important factor to consider, as is battery life.
Long-term care. For many patients in long-term care environments, a ventilator with basic ventilation modes is sufficient to meet their needs, and extra features that contribute to greater complexity and higher price are not desirable. The devices we evaluated for long-term care are more advanced (and expensive) than those commonly used in this setting, although they are acceptable for use in this environment, and there may be instances in which the advanced features would be desirable. When considering devices for long-term care, pay attention to the alarm capabilities of the device. Because long-term care environments are not as highly staffed as an intensive care unit (ICU), alarms that can be detected quickly and understood easily are essential. Similarly, the ability to pass alarm information to a remote system is also important.
Mass-casualty critical care. This is an emerging application for portable/transport ventilators. In certain mass-casualty scenarios, such as a pandemic or a biological terrorist attack, a large number of patients may experience severe and complex lung damage that requires respiratory support for several days.
For this scenario, it is expected that patients will have symptoms similar to those of acute respiratory distress syndrome and that facilities may not have reliable electricity or medical gas supplies. Thus, to adequately fill this niche, ventilators at minimum should have independent controls for tidal volume, fraction of inspired oxygen (FiO2), respiratory rate (RR), and positive end-expiratory pressure (PEEP); should be able to operate on external battery for four hours (adult settings); should have minimal oxygen consumption; should have some method (e.g., a turbine) of operating without any compressed gas source; and should accept both 50 psi and low-flow oxygen. Also, the ventilators should be able to ventilate pediatric and adult patients. Price is obviously very important as well, since a less expensive device allows an organization to amass a larger stockpile with the same budget. (For more on stockpiling strategies, see the discussion Stockpiling for Mass-Casualty Events below.) Because skilled clinicians may be overwhelmed by patients in a mass-casualty scenario, devices should be easy to use for minimally trained users.
Although there are some mass-casualty scenarios in which ventilators may be used outside of a medical facility, for the purposes of this Evaluation we only considered requirements for devices used in medical facilities (mobile critical care medical facilities, veterinary hospitals, outpatient surgical procedure centers, and locations inside hospitals, including ICUs, postanesthesia care units, emergency departments, intermediate care and step-down units, large procedure suites, telemetry units, and general care wards).
Prehospital and between-hospital transport applications are not considered in this Evaluation.
_________________________________________________________
The Bottom Line
Many of today’s portable/transport ventilators have a full complement of advanced features, and can even serve as a department’s primary ventilator. However, advanced settings may go unused in some settings (e.g., long-term care). It is important to be aware of the needs of the expected patient population and pick a model to match.
- Models were rated as follows:
— For in-hospital transport, we rated the Hamilton Medical C2 highest (four out of five stars). The CareFusion LTV 1200, Draeger Oxylog 3000, and GE Healthcare/VersaMed iVent201received three stars.
— For long-term care and mass-casualty critical care, most models were average. For both applications, the CareFusion LTV 1200, Draeger Carina, and GE Healthcare/VersaMed iVent201 earned three out of five stars; the Hamilton Medical C2, however, received only two stars.
- We also evaluated one ventilator designed specifically for mass-casualty critical care: the Allied MCV100. This unit may be considered as a supplemental unit in a “mixed” approach to mass-casualty stockpiling, in which full-featured models and less expensive supplemental units are used together in order to meet the needs of a full range of patients while reducing costs. However, because this unit has several drawbacks, it receives only a two-star rating, even for this limited application.
_________________________________________________________
Ratings—In-Hospital Transport
A summary of our findings is provided below. The product profiles below contain our complete findings, including detailed test results that may further help in making a purchasing decision.
Products with the same rating are listed alphabetically.
Hamilton Medical C2

Capabilities—Excellent
Portability—Fair
Battery life—Excellent
The Hamilton Medical C2 is a very good ventilator for this application. It has almost all the features found in today’s top-of-the-line ICU ventilators, making it possible to transport almost any patient without sacrificing ventilatory support. In addition, the C2 has excellent internal battery life and a large color touchscreen display that allows easy control of settings and presents important information in easy-to-understand visuals. The main disadvantage is that this device is large and heavy, making it unrealistic to expect clinicians to carry the device during transport. Mounting this ventilator on its cart or to the bed is more practical. This is also the most expensive unit we evaluated.
CareFusion LTV 1200

Capabilities—Good
Portability—Excellent
Battery life—Good
The CareFusion LTV 1200 has some features beyond the minimal feature set of the other three-star units, but these additional features are of limited utility. The display can show only a limited amount of information at once, and accessing some of the settings and features requires using the extended menu, which is complicated. However, the unit is small and relatively lightweight, and a magnetic resonance (MR)-conditional configuration is available.
Draeger Oxylog 3000

Capabilities—Good
Portability—Good
Battery life—Excellent
The Draeger Oxylog 3000 is slightly smaller and lighter than the other three-star units, but it is the only one of the three that requires a 50 psi source (e.g., oxygen tank) to ventilate the patient; the other units can also operate from internal air turbines to allow ventilation if the oxygen tank runs out.
GE Healthcare/VersaMed iVent201

Capabilities—Good
Portability—Fair
Battery life—Good
The GE Healthcare/VersaMed iVent201 has the largest display of the three-star devices, making it easy to use, but it is large and heavy. An MR-conditional configuration of this device is available. One problem we noted with the iVent201 is that maximum flow is limited when PEEP is set to zero; this may be a concern for some patients and may confuse clinicians. Users must be aware of this limitation and how to deal with it.
Not rated: Draeger Carina (This device is not marketed for this application.)
Ratings—Long-Term Care
Any of the three-star devices would be an acceptable choice for long-term care settings; however, they are all relatively expensive compared to other models (not evaluated by ECRI Institute) that are marketed for this application. Facilities should consider those less expensive units when making a purchasing decision.
CareFusion LTV 1200

Alarms—Good
Price—Fair
The CareFusion LTV 1200 has a steep learning curve, and its alarms are sometimes difficult to identify. Other models from the LTV product line (e.g., LTV 1150) might be a better choice for this care environment due to their lower prices.
Draeger Carina

Alarms—Good
Price—Fair
Draeger’s Carina is easy to use, it has effective onscreen alarm notification, and it is the most compact long-term care unit we evaluated. However, PEEP cannot be set below 3 cm H2O. The device has several features that purport to facilitate the use of noninvasive ventilation (NIV), although we did not evaluate the effectiveness of those features.
GE Healthcare/VersaMed iVent201

Alarms—Good
Price—Fair
The GE Healthcare/VersaMed iVent201 has the largest display of the three-star devices. One problem we noted with the device is that maximum flow is limited when PEEP is set to zero; this may be a concern for some patients and may confuse clinicians. Users must be aware of this limitation and how to deal with it.
Hamilton Medical C2

Alarms—Excellent
Price—Poor
The very high price of the Hamilton Medical C2 (significantly more than the next highest unit) makes it less desirable for long-term care applications. The advanced features provided by the C2 (discussed below) are not necessary for most long-term care patients, but might be useful in some situations. On the plus side, the dome light on top of the C2 makes the alarms noticeable from very far away, helping to alert caregivers who may not be in the immediate vicinity of the patient.
Not rated: Draeger Oxylog 3000 (This device is not marketed for this application.)
Ratings—Mass-Casualty Critical Care
None of the units we tested are ideal choices for this application. Although prices vary depending on the number of units being purchased, each of the three-star models is priced such that acquiring a large number of devices for a stockpile is not likely to be within the budget of any single facility. The two-star unit is even more expensive and also uses oxygen inefficiently.
CareFusion LTV 1200

Oxygen—Fair
Ease of use—Fair
Price—Fair
The CareFusion LTV 1200’s oxygen consumption in the default mode is well beyond the acceptable range specified in our criteria. If the O2 Conserve feature is turned on, the device is very efficient with oxygen use. However, activating this mode requires navigating the extended menu each time the device is set up for a patient. The need to enable this mode is not obvious and the steps required to do so are relatively complicated. Therefore, this requirement may be easily overlooked, especially in an emergency situation. This makes it essential for users in a situation where oxygen supplies may be limited to be properly trained in setting the unit to the O2 Conserve mode. Other disadvantages are that the small display can present only a limited amount of information, and the extended menu is complicated.
Draeger Carina

Oxygen—Good
Ease of use—Good
Price—Fair
The Draeger Carina is a satisfactory unit for this application, with no outstanding advantages or disadvantages.
GE Healthcare/VersaMed iVent201

Oxygen—Good
Ease of use—Good
Price—Fair
The GE Healthcare/VersaMed iVent201’s Adaptive Flow and Adaptive I-Time features, when used together, can make it easier for minimally trained users to set up the device.
Hamilton Medical C2

Oxygen—Fair
Ease of use—Good
Price—Poor
The C2 is not a good choice for mass-casualty applications because it does not use oxygen efficiently (in fact, it is extremely inefficient at 100% oxygen; see the C2's product profile) and is the most expensive device we evaluated. On the other hand, the display communicates information very clearly and the ventilator performed very well in our battery testing.
Not rated: Draeger Oxylog 3000 (This device is not marketed for use in mass-casualty critical care applications.)
`
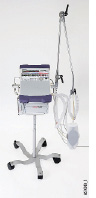 CareFusion Pulmonetic Systems LTV 1200
CareFusion Pulmonetic Systems LTV 1200
CareFusion Corp. Ventilators Div. [454433], Minneapolis, MN (USA); +1 (800) 520-4368, +1 (763) 398-8300; www.viasyshealthcare.com
In-hospital transport. 
Long-term care. 
Mass-casualty critical care. 
Performance Judgments
In-hospital transport
Capabilities—Good
Portability—Excellent
Battery life—Good
Long-term care
Alarms—Good
Price—Fair
Mass-casualty critical care
Oxygen—Fair
Ease of use—Fair
Price—Fair
Product Description
The LTV 1200 is the latest in a long line of LTV ventilators. It is distinguished from its popular predecessor, the LTV 1000, by the addition of internal control of PEEP. It can be used for in-hospital transport, long-term care, and mass-casualty critical care. (It also can be used in home care and for pre- and between-hospital transport, though these applications are not covered in this article.) Like the LTV 1000, the LTV 1200 can operate with both low-flow and high-pressure oxygen, and both units have a dedicated single-limb circuit with a pneumatically operated exhalation valve and a flow sensor at the proximal end of the circuit. The display on the device is a relatively small LED array. The LTV 1200 can deliver tidal volumes from 50 to 2,000 mL. Our testing was performed on a device running software version 05.06.
Introduced in 2006, the CareFusion LTV 1200 is marketed worldwide.
- Dimensions (H × W × D): 25 × 30 × 8 cm (9.8 × 11.8 × 3.2 in)
- Weight: 6.5 kg (14.5 lb)
- List prices:
— LTV 1200: $16,100
— Graphical monitor: $4,120
- Manufacturer-recommended maintenance interval: 1 year
Significant Test Results
The LTV 1200 would be an acceptable choice for any of the three applications considered in this Evaluation.
This unit had only fair oxygen consumption (well beyond the acceptable range specified in our criteria) in its normal (default) operating oxygen mode. However, with the O2 Conserve feature turned on, the unit had excellent oxygen consumption, better than any other evaluated unit. This feature, which is accessed from the extended menu, changes the LTV 1200 from the default flow-triggering setting (which requires a small bias flow in the circuit at all times) to pressure triggering (which does not require a bias flow) and must be activated every time the unit is set up for a patient.
Among this unit’s advantages are internal control of PEEP, adjustable rise time, and the ability to be used with both low-flow and high-pressure oxygen sources. Additionally, the unit comes with an adapter that allows it to be powered by a 12-volt power source. An MR-conditional configuration of the LTV 1200 is also available.
| |

|
| |
Incomplete connection. The LTV 1200’s high-pressure oxygen inlet is very close to the chain that connects to the inlet’s cap. Larger thumb-tightening connectors on certain hoses, like the one shown at left, can only be partially advanced along the inlet’s threads before running up against the chain, preventing sufficient tightening. A traditional connector, which can be tightened fully, is shown at right. |
One drawback is that the LTV 1200 has a steep learning curve (which would be especially problematic for users in mass-casualty scenarios, who would be less likely to be familiar with the device). In particular, navigating the extended menu is challenging, mainly due to the small display (an array of LEDs), which limits the amount of information that can be simultaneously displayed, and the limited controls for extended-menu navigation (one button and one knob). Although basic settings can be changed relatively easily using the unit’s buttons and control knob, accessing many features and alarms requires the user to access and navigate the extended menu. Furthermore, during an alarm condition, the displayed alarm takes up the whole screen; all the values that are normally displayed are hidden from the user, and the extended menu cannot be accessed.
An additional design drawback is that hoses with larger thumb-tightening connectors cannot be fully connected to the high-pressure oxygen inlet. These connectors can only be partially advanced along the inlet’s threads before running up against the chain that attaches the cap to the inlet, preventing sufficient tightening (see the photos). Also, because the unit is tall and narrow, it is prone to tipping. However, the manufacturer does not recommend placing the unit upright without a mounting bracket, and the unit will operate from any orientation.
During our testing, the internal battery lasted an hour and a half. This is acceptable for long-term care and in-hospital transport ventilators, but well below the requirement for mass-casualty devices.
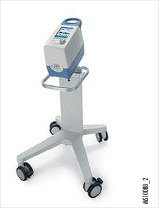 Draeger Carina
Draeger Carina
Draeger Medical, Inc. [371341], Telford, PA (USA); +1 (800) 437-2437, +1 (215) 721-5400; www.draegermedical.com
In-hospital transport. Not marketed for this application
Long-term care. 
Mass-casualty critical care. 
Performance Judgments
In-hospital transport
Not marketed for this application
Long-term care
Alarms—Good
Price—Fair
Mass-casualty critical care
Oxygen—Good
Ease of use—Good
Price—Fair
Product Description
The Carina can be used with one of two dedicated single-limb circuits. The LeakV circuit allows a constant flow of air out of the leak valve, which enables exhaled gases to be passively vented to the atmosphere between breaths. The ExpV circuit has an expiratory valve that is pneumatically activated by the ventilator via a small secondary lumen. The LeakV circuit can be used for both intubated patients and NIV, while the ExpV circuit can only be used with intubated patients.
The Carina can deliver tidal volumes from 100 to 2,000 mL and PEEP from 3 to 20 cm H2O. The Carina has AutoFlow, Draeger’s combination mode, and SyncPlus, an advanced trigger-sensing algorithm that adapts to the patient’s spontaneous respiratory cycle and uses multiple detection criteria to reduce missed triggers. We did not evaluate SyncPlus.
Our testing was performed on a device running software version 3.11, and with both LeakV and ExpV circuits (except where otherwise noted).
Introduced in April 2009, the Draeger Carina is marketed worldwide.
- Dimensions (H × W × D): 17.5 × 27.5 × 38.5 cm (6.9 × 10.8 × 15.2 in)
- Weight: 4.9 kg (10.8 lb)
- List price: $16,250
- Manufacturer-recommended maintenance interval: 1 year
Significant Test Results
The system is an acceptable choice for either a mass-casualty scenario or the long-term care environment.
The Carina has a relatively large (13.7 cm/5.4 in diagonal) color display, which facilitates setup and makes viewing alarm and other information and operating the device very easy. This unit comes with pressure-control breaths, has adjustable rise time and internal control of PEEP, and can be used with both low-flow and high-pressure oxygen sources. When the LeakV circuit is used, this unit automatically compensates for leakage and displays calculated inspiratory volumes. It can also operate in any orientation and is resistant to tipping. The unit has several features that purport to facilitate use of NIV (although we did not evaluate the effectiveness of those features).
One disadvantage is that the Carina does not use oxygen as efficiently as some of the other evaluated devices. Note that all oxygen consumption testing was performed with the ExpV circuit (since the LeakV circuit has a constant flow to allow passive clearance of exhaled gas, which significantly increases oxygen consumption). Also, this unit cannot be used with infants and some pediatric patients because the minimum tidal volume it can deliver is 100 mL.
The Carina’s internal battery lasted a little more than an hour. This is acceptable for long-term care and in-hospital transport ventilators, but well below the requirement for mass-casualty devices.
 Draeger Oxylog 3000
Draeger Oxylog 3000
Draeger Medical, Inc. [371341], Telford, PA (USA); +1 (800) 437-2437, +1 (215) 721-5400; www.draegermedical.com
In-hospital transport. 
Long-term care. Not marketed for this application
Mass-casualty critical care. Not marketed for this application
Performance Judgments
In-hospital transport
Capabilities—Good
Portability—Good
Battery life—Excellent
Long-term care
Not marketed for this application
Mass-casualty critical care
Not marketed for this application
Product Description
The Oxylog 3000 is designed for transporting patients within the hospital as well as outside the hospital (i.e., pre- and between-hospital transport). It has a dedicated single-limb circuit with a pneumatically operated expiratory valve. This unit can deliver tidal volumes from 50 to 2,000 mL.
Our testing was performed on devices running software version 01.11 and 01.12.
Introduced in 2006, the Draeger Oxylog 3000 is marketed worldwide.
- Dimensions (H × W × D): 18.4 × 28.2 × 17.5 cm (7.2 × 11.1 × 6.9 in)
- Weight: 5.4 kg (11.9 lb)
- List price: $17,150
- Manufacturer-recommended maintenance interval: 2 years
Significant Test Results
The Oxylog 3000’s graphical display, long internal battery life, and numerous modes all make this an acceptable ventilator for in-hospital transport.
The Oxylog 3000 has several advantages. The control knobs make it easy to set key values such as tidal volume, frequency, maximum inspiratory pressure, and oxygen concentration. Battery life is good; the internal battery lasted three-and-a-half hours during our testing. In addition, this unit has internal control of PEEP. It can also be operated in a variety of orientations and is resistant to tipping. It has a built-in hook for attaching to a bedrail or a fixture in a vehicle. An optional converter allows the device to be run from a 12-volt source. Also, the unit’s recommended maintenance interval (two years) is longer than that of the other evaluated devices, easing the maintenance burden on the clinical engineering department.
Among the unit’s disadvantages, our primary concern is that it must be connected to a high-pressure (50 psi) gas source (typically oxygen) in order to operate. If no high-pressure source is available, the device will not deliver breaths; other ventilators allow the user to continue ventilation with air. While a high-pressure oxygen source (tank) is generally available in a transport situation, users will need to have a plan for what to do if it is depleted. Also, FiO2 cannot be set below 40%; although this will not be a problem in most situations, there are some patients who require less than 40% oxygen.
Other disadvantages are that the device does not allow the user to set flow termination for pressure-supported breaths and does not automatically compensate for the compliance of the circuit. Also, there is no lockout feature for the control knobs, and changing them does not require confirmation. And the unit’s oxygen connection is inconveniently placed, making connecting and disconnecting hoses awkward.
 GE Healthcare/VersaMed Medical Systems iVent201
GE Healthcare/VersaMed Medical Systems iVent201
GE Helthcare UK Ltd. [401906], Chalfont St Giles Buckinghamshire (England); (800) 0329201, 44 (870) 6061920; http://www.gehealthcare.co.uk/
In-hospital transport. 
Long-term care.
Mass-casualty critical care.
Performance Judgments
In-hospital transport
Capabilities—Good
Portability—Fair
Battery life—Good
Long-term care
Alarms—Good
Price—Fair
Mass-casualty critical care
Oxygen—Good
Ease of use—Good
Price—Fair
Product Description
GE Healthcare/VersaMed’s iVent201 is a portable ventilator that can be used with adult and pediatric patients, and is able to deliver tidal volumes as small as 50 mL. It comes in several different configurations. All iVent201 configurations have Adaptive Peak Flow and Adaptive I-Time, two features that work together in volume-control modes to maintain an inspiratory/expiratory (I:E) ratio of 1:2 while responding to changes in the patient’s spontaneous effort and demand.
Our testing was performed on an iVent201 IC running software version 19.18.01 (012). Because VersaMed markets different iVent201 configurations for different applications, we have based our judgments on the most appropriate configuration, as noted below. Although we did not test these other configurations, VersaMed states that the differences between the configurations would not affect our test results.
Introduced in 2001, the iVent201 is marketed worldwide.
- Dimensions (H × W × D): 33 × 24 × 26 cm (13 × 9.5 × 10.3 in)
- Weight: 11 kg (24 lb)
- List price:
— iVent201 IC (appropriate for in-hospital transport, has most comprehensive set of modes and breath types among the available iVent201 configurations as well as all graphical capabilities): $19,995
— iVent201 SA (appropriate for long-term care, has both volume and pressure breaths as well as bilevel mode, graphics lack pulmonary mechanics and loops): $17,950
— iVent201 DHHS (appropriate for mass-casualty critical care, unique color combination to distinguish stockpiled devices from normal fleet): $11,545
- Manufacturer-recommended maintenance interval: 1 year
Significant Test Results
The GE Healthcare/VersaMed iVent201 would be an acceptable choice for any of the applications we are considering. Clear presentation of information on the color display makes the device easy to use. Also contributing to ease of use are the Adaptive Flow and Adaptive I-Time features, which make it easier for nonexpert users to set up the device. The unit also has internal control of PEEP, which is adjustable up to 40 cm H2O (this may be an advantage for some patients, compared to the 20 cm H2O limit of other units); adjustable rise time; and both high-pressure and low-flow oxygen inlets. The device comes with an adapter that allows it to be powered by a 12-volt power source.
The iVent201 uses oxygen relatively efficiently. Additionally, an MR-conditional configuration of the iVent201 is available.
A disadvantage is that the iVent201 was not able to reach higher pressures or tidal volumes when PEEP was set to zero; this may be a concern for some patients and may cause confusion. Users must be aware of this limitation and how to deal with it. Also, at 24 lb, the iVent201 is one of the larger and heavier units we tested.
The internal battery lasted for about an hour and a half. This is acceptable for long-term care and in-hospital transport ventilators, but well below the requirement for mass-casualty devices.
`
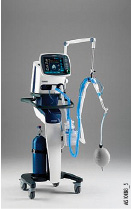 Hamilton Medical C2
Hamilton Medical C2
Hamilton Medical, Inc. [105689], Reno, NV (USA); +1 (775) 858-3200, +1 (800) 426-6331; www.hamilton-medical.com
In-hospital transport. 
Long-term care.
Mass-casualty critical care.
Performance Judgments
In-hospital transport
Capabilities—Excellent
Portability—Fair
Battery life—Excellent
Long-term care
Alarms—Excellent
Price—Poor
Mass-casualty critical care
Oxygen—Fair
Ease of use—Good
Price—Poor
Product Description
Hamilton Medical refers to the C2 as a “compact ICU ventilator,” although it is also intended for use in subacute and in-hospital transport applications. It can be used with adult and pediatric patients, and is able to deliver tidal volumes as low as 20 mL. The C2’s features include IntelliTrig and Adaptive Support Ventilation (ASV). IntelliTrig is an algorithm that detects changes in leaks during NIV and adjusts trigger sensitivity accordingly. ASV is a patient-responsive mode that monitors the patient’s spontaneous activity and lung mechanics and uses that information to automatically change between control and support modes as well as to adjust settings in those modes. Hamilton Medical claims that ASV requires fewer clinician interactions with the device than other ventilation modes. We did not evaluate IntelliTrig or ASV.
Our testing was performed on a device running software version 1.1.0.
Introduced in 2008, the Hamilton Medical C2 is marketed worldwide.
- Dimensions (H × W × D): 43 × 33 × 31 cm (16.9 × 13 × 12.2 in)
- Weight: 9.5 kg (21 lb)
- List price: $30,000
- Manufacturer-recommended maintenance interval: 1 year
Significant Test Results `
The Hamilton Medical C2 is a very capable portable ventilator with features comparable to many ICU ventilators. This unit has all the required and preferred features listed in our criteria. It has a large and easy-to-use touchscreen display. In addition to ASV, this device includes Dynamic Lung (a visual model of the lung that provides a real-time graphical representation of vital information—tidal volume, lung compliance and resistance, and spontaneous patient effort—in an easy-to-understand manner) and combination mode. Also, the internal batteries lasted more than four hours—longer than any other evaluated device. There is also a dome light on the top of the unit (indicating the priority of the alarm) that can be seen 360° around the device. The C2 has internal control of PEEP, which is adjustable up to 35 cm H2O (this may be an advantage for some patients, compared to the 20 cm H2O limit of other units); has adjustable rise time; and can be used with both low-flow and high-pressure (50 psi) oxygen sources.
A disadvantage is that the C2 did not use oxygen efficiently in our testing. At similar settings, the unit consumed oxygen more quickly than some of the other units. In addition, at an FiO2 of 100%, the C2 behaves unusually: It draws twice as much oxygen from the high-pressure inlet as required. For example, for the 100%-16 L/min test (see the description of our oxygen consumption testing below), the device actually used 32 liters of oxygen every minute (the delivered tidal volumes were not affected). This problem is avoided if the FiO2 is set just below 100% (e.g., 99%), but users are not likely to be aware of this. (Note that because of this problem, our 100% oxygen testing was actually performed at 99% for this unit.) Other drawbacks are that the C2 is large and heavy (21 lb), and cannot be operated in more than one orientation. It is also much more expensive than the other devices we evaluated.
Stockpiling for Mass-Casualty Events `
Issues
In the wake of a natural disaster, terrorist attack, or other mass-casualty event, there is likely to be a surge in the number of patients who require ventilatory support. Concerned about this possibility, some organizations are considering stockpiling ventilators for use in mass-casualty situations.
In an ideal world, stockpiled ventilators would offer a wide range of features and capabilities. They would be able to effectively ventilate all (or nearly all) victims of a mass-casualty event, be extremely easy to use so that individuals without formal clinical schooling or experience could operate them with minimal instruction or attention, and be very inexpensive so that they could be economically purchased in large quantities (in addition to meeting other criteria that we have spelled out for the units in this Evaluation).
Unfortunately, such an ideal ventilator does not exist. Performance- and capability-rich ventilators will generally be more expensive and more difficult to use, whereas simpler, less expensive units will not be able to provide effective ventilation over the full range of circumstances and victims that may be encountered.
Faced with this reality, some organizations may elect to assemble a mixed stockpile consisting of two different types of ventilators—what might be called full-featured mass-casualty units and supplemental mass-casualty units.
- Full-featured mass-casualty units are more expensive, more complex portable ventilators that will meet most expected needs in a mass-casualty situation. Most portable ventilator models fall into this category.
- Supplemental mass-casualty units are a relatively new type of ventilator designed specifically for mass-casualty applications. These units are far less expensive than full-featured units and are also easier to operate, allowing use by staff with less training. These units may not meet a full range of needs, but their lower cost allows more units to be purchased for situations in which the need for quantity becomes overwhelming. We are aware of two manufacturers that offer supplemental mass-casualty ventilators: Allied Healthcare and Impact Instrumentation.
Supplemental units should be used only in conjunction with an inventory of more capable units. Organizations purchasing them must recognize that these devices cannot make up the entire stockpile. Because of their limitations, they are unsuitable for some portion of the expected patient population. It is therefore crucial that the organization have a plan in place to make sure that, in the chaos of a mass-casualty scenario, these devices do not end up on patients they cannot support.
We have evaluated one supplemental mass-casualty unit—the Allied Healthcare MCV100.
Evaluation of the Allied Healthcare MCV100 `
The MCV100 is designed specifically as a mass-casualty ventilator for what Allied calls an “overwhelming surge,” in which the number of affected patients far surpasses the available respiratory care staff. It is a very basic ventilator, with controls for tidal volume, respiratory rate, and gas source, as well as high- and low-airway-pressure alarms. The user can also control the setting for the airway pressure-relief valve. This device does not have internal control of PEEP, instead requiring the use of an external PEEP valve. The MCV100 can deliver tidal volumes from 200 to 1,200 mL. This model comes in two configurations. The basic configuration allows the user to select between air (from the internal compressors) and oxygen (from the high-pressure inlet). The second configuration (MCV100-B) has an additional setting, 60% oxygen.
Because the MCV100 is not intended to be on a par with traditional ventilators, many of the criteria we use to evaluate traditional ventilators (such as the other models in this Evaluation) cannot be applied. Therefore, we have used a less stringent set of criteria, specific to supplemental mass-casualty units, to evaluate the MCV100.
No photo was available for publication.
Rating (as a supplemental mass-casualty ventilator)

Oxygen—Fair
Ease of use—Excellent
Price—Good
For facilities that are looking at a mix of devices to meet ventilation requirements for mass-casualty critical care, the MCV100 can be considered as a supplement to a more capable model. Its advantages for this application are that the simple nature of the device makes it very easy to use, the internal battery lasts a long time (more than four hours during our testing), and the oxygen consumption is good. Also, the price is much lower compared to the other devices we evaluated.
A major disadvantage is the failure to provide prominent alarms for critical events. The alarm volume is too quiet and cannot be adjusted, and the unit does not have different-priority alarms. The visible alarm is a small and relatively dim LED. This problem is a concern in all applications and contributes to our giving the device only a two-star rating, even for this supplemental role.
There are also flow concerns. The maximum flow that can be generated by this unit is 36 L/min (although the device is designed so that the patient can draw in additional ambient air during spontaneous breathing). This is insufficient for larger patients who are breathing spontaneously and falls well short of acceptable levels. In addition, the smallest tidal volume this device can deliver is 200 mL, which is too large for all neonates and many pediatric patients.
Other concerns are that the power button does not prevent inadvertent shutoff and that there is no way to prevent inadvertent setting changes. Also, during our testing, a safety valve failed, causing the oxygen from the connected E-cylinder to vent to atmosphere even when the device was powered off.
It is worth noting that the MCV100 does not have a true internal blender that would allow the user to set oxygen concentration anywhere between 21% and 100%; this model can only deliver air or oxygen, making it inappropriate for a number of patients. However, the MCV100-B, which allows the user to choose between 21%, 60%, and 100% oxygen, is an acceptable option.
This unit performed adequately in our oxygen consumption tests (see this table).
Facilities that use this model should develop guidelines for its deployment to ensure that it is used only as a supplemental unit. The MCV100 is unsuitable for use as the main ventilator for mass-casualty applications, due mainly to the limited range of patients for whom it can be used, the difficulty in noticing its alarms, and the limitations of its modes and features that might be called for in this application (e.g., lack of SIMV and CPAP).
Product Description
Supplier. Allied Healthcare Products, Inc. [105171], St. Louis, MO (USA); +1 (800) 444-3954, +1 (314) 771-2400; www.alliedhpi.com
Availability. Introduced in 2008; marketed worldwide (except in Asia)
Version we tested. MCV100-B
Specifications
- Dimensions (H × W × D): 29.2 × 26.1 × 8.9 cm (11.5 × 10.3 × 3.5 in)
- Weight: 6.3 kg (14 lb)
- List price: $2,835 (MCV100); $3,532 (MCV100-B)
- Manufacturer-recommended maintenance interval: 1 year
Evaluation Criteria and Test Methods
We based our criteria and test methods on perspectives of our clinical reviewers and the resources listed below.
ECRI Institute’s previous ventilator Evaluations:
The following standards and guidelines:
- American Association for Respiratory Care. Guidelines for Acquisition of Ventilators to Meet Demands for Pandemic Flu and Mass Casualty Incidents (May 2006).
- ASTM International. Standard Specification for Electrically Powered Home Care Ventilators, Part 1—Positive-Pressure Ventilators and Ventilator Circuits. ASTM F1246-91 (2005).
- Definitive Care for the Critically Ill during a Disaster: Medical Resources for Surge Capacity. From a Task Force for Mass Critical Care Summit Meeting, January 26-27, 2007, Chicago, IL. Chest, Vol. 133, May 2008 (Supplement).
- International Organization for Standardization (ISO):
— Lung Ventilators for Medical Use—Particular Requirements for Basic Safety and Essential Performance—Part 2: Home Care Ventilators for Ventilator-Dependent Patients. ISO 10651-2:2004.
— Lung Ventilators for Medical Use—Part 3: Particular Requirements for Emergency and Transport Ventilators. ISO 10651-3:1997.
— Lung Ventilators for Medical Use—Particular Requirements for Basic Safety and Essential Performance—Part 6: Home-Care Ventilatory Support Devices. ISO 10651-6:2004.
The “Key Ventilator Criteria” table lists key criteria and required and preferred capabilities for ventilators intended to be used in long-term care, in-hospital transport, and mass-casualty applications.
Performance
Functionality
Criteria
Devices used in mass-casualty critical care applications should be able to provide flow between 10 and 80 L/min. For additional criteria, see the “Key Ventilator Criteria” table. Advanced or additional features may be advantageous in some circumstances. (We note these for specific units in the product profiles.)
Accuracy
Criteria
The primary ventilation variables delivered should be within 10% of the set values; such variables include (1) tidal volume (after we corrected for breathing circuit compliance to compensate for the volume of gas in the breathing circuit), (2) pressure-control level (if available), (3) respiratory rate, and (4) I:E ratio or inspiratory time. The ventilator should meet this criterion over a range of typical settings (including PEEP settings up to 15 cm H2O) without generating inadvertent PEEP.
If the unit has an integral oxygen blender, oxygen-air mixtures should be accurate to within 4% across a variety of FiO2 settings (i.e., a unit set to deliver an FiO2 of 50% should deliver somewhere between 46% and 54%).
Test method. The following test method was used for all tests in which we checked ventilator performance and accuracy. Each unit was tested for its ability to meet our ventilation test conditions (see the “Ventilation Test Conditions” table) without causing inadvertent PEEP. In each case, the I:E ratio was 1:2. Lung compliances were simulated with a Michigan Instruments Vent-Aid TTL test lung (for adult lung conditions) and bottles of known compliance (for infant and pediatric lung conditions), and parabolic flow resistors were used to simulate airway resistance. We measured pressures, flows, and volume at the wye connection of the breathing circuit with a gas waveform analyzer developed at ECRI Institute. The analyzer recorded and digitally stored waveforms for all of these variables. We tested the inspired oxygen concentration control by connecting the ventilator to an oxygen source. FiO2 was set at 0.6 and 0.9. Pediatric and infant configurations were tested with no PEEP. Adult configurations were tested at PEEP levels of 0 and 15 cm H2O. We verified that the ventilator was able to satisfy the flow requirements in the “Key Ventilator Criteria” table.
Battery and Other Power Sources
Criteria
- All units should have provisions for an internal battery and a connection for an external battery.
- To conserve battery power, the ventilator should automatically switch to alternating-current (AC) power whenever the device is connected to line power. If AC power is lost, the ventilator should automatically switch to the external battery (if connected). If the external battery falls below the required voltage (or if no external battery is connected), the ventilator should automatically switch to its internal battery. Ventilation should not be interrupted when the ventilator switches between power sources.
- The ventilator should visually indicate the power source in use.
- An internal battery that is defective (e.g., short-circuited cell) or depleted should not inhibit the ventilator’s operation from an AC source.
- We prefer that the ventilator activate a periodic audible advisory while operating on its internal battery to remind the user that the ventilator is operating on its emergency backup power supply.
- It should be possible to operate the ventilator from its internal battery if the external battery is connected in reverse polarity, and the ventilator should operate from the external battery immediately after the leads are reconnected correctly.
- The internal battery should charge automatically whenever the ventilator is connected to AC power.
- A visual indicator should identify when the ventilator is charging.
- The battery charge indicator should accurately reflect battery power available.
Test method. We inspected and operated the ventilators to check for compliance with these criteria. For mass-casualty applications, the test conditions were as follows: A/C, volume breaths, 450 L tidal volume, 10 cm H2O PEEP, 35 breaths/min, 1:2 I:E ratio, 15 mL/cm H2O compliance, 20 cm H2O/L/sec resistance, no compressed gas source. Test conditions for other applications were as follows: A/C, volume breaths, 20 breaths/min, 500 L tidal volume, standard test lung, 5 cm H2O PEEP, no compressed gas source. Because an actual short circuit is highly unlikely, rather than short-circuiting the battery leads, we completely depleted the battery and then operated the device on AC power.
We observed the ventilator while it operated on its internal battery under the adult ventilation conditions and verified its performance. We ran the unit at the conditions described above until the battery was depleted.
Oxygen Consumption `
Criterion. For mass-casualty scenarios, when delivering a minute volume of 16 L/min, the unit should operate on an E tank for at least 38 minutes at an oxygen setting of 100% and at least 104 minutes at an oxygen setting of 50%. When delivering a minute volume of 6 L/min, the unit should operate on an E tank for at least 100 minutes at an oxygen setting of 100% and 280 minutes at an oxygen setting of 50%.
Test method. We tested each device at the listed settings.
Note: The criterion above was taken directly from the Task Force for Mass Casualty Critical Care’s “Definitive Care for the Critically Ill During a Disaster: Medical Resources for Surge Capacity.” The criterion requires highly efficient use of compressed oxygen. For reference, given a full E tank (660 L), a perfectly efficient ventilator would operate as shown in the "Optimal Oxygen Consumption" table.
It should be noted, however, that several factors can affect operating time, such as true fill volume (suppliers may fill tanks to more than the nominal volume); different low-pressure thresholds among devices, which leads to different amounts of residual oxygen in “empty” tanks; and slight inaccuracies in delivered tidal volume and FiO2.
Alarms and Safety Features
The ventilator’s alarms notify the user of problems with ventilator function and possible changes in the patient’s condition. A remote alarm can be connected to the ventilator to convey an audible warning to a clinician who is away from the ventilator. The features of the alarm system and its ease of use are important to provide the information required for a prompt, appropriate response.
Alarm Conditions
Criteria
The unit should have alarms for the following conditions:
Low Volume
The unit should have a low-volume alarm, either tidal or minute volume.
Circuit Disconnect
The unit should have an automatic alarm that detects a breach of breathing circuit integrity that results in more than 10% loss in delivered volume.
High and Low Inspiratory Pressure
Apnea
The unit should alarm if the patient stops breathing. The apnea threshold should be clinician-adjustable, but the upper limit should not exceed 60 seconds.
Alarm Characteristics
Criteria
Identification
- Identifying the cause of an alarm should be easy in order to allow quick assessment and correction of the alarm condition.
- The priority of the alarm condition should be indicated by different audible tones and different-colored visual indicators.
- At any volume setting, alarms should be audible over and distinct from operating sound levels.
- Visual indicators should be prominent and easy to see in various types and intensities of ambient light, at distances of three feet from the display, and at viewing angles of at least 60° from perpendicular to the plane of the display.
- In addition to visual indicators for specific alarms, we prefer that ventilators include a general visual indicator that can be seen clearly from at least 15 feet away in any direction to warn clinicians that an alarm condition exists. This type of indicator is intended to draw attention to life-threatening conditions; therefore, at minimum, it should activate with high-priority alarms.
Activation
- Alarms should activate immediately when an alarm setting is exceeded or when a condition is detected, except that delays for high-pressure alarms are permissible.
- When an alarm limit is exceeded, the device should activate an audible alarm and a visual indicator and continue to alarm as long as the value is outside the limit.
- Visual indicators should remain lit, even after a condition has been corrected or is no longer present, until they are manually reset.
Disabling and Silencing
- Disabling alarms should not be possible.
- It should be possible to temporarily silence audible alarms; however, the alarm should reactivate within two minutes. A visual alarm-silence indicator should be active for the duration of the silence period.
- If a second alarm condition occurs while an alarm is silenced, it should be brought to the user’s attention with another audible alarm and a visual indicator.
Alarm Limits
- We prefer that the ventilator have an automatic alarm-setting feature that sets the low-pressure alarm at 5 to 7 cm H2O below the patient’s peak inspiratory pressure and that sets the low-minute-volume alarm at 10% to 15% below the patient’s minute volume.
- Setting alarm limits should be quick and easy.
- We prefer that alarm limits be displayed continuously.
- Alarm limits should not be adjustable beyond reasonable clinical limits.
Battery-Power Alarms
- An audible alarm and visual indicator should activate when the ventilator switches from line to battery power and from the external battery to the internal battery. The audible alarm should sound until manually reset to ensure that the user has been notified of the change in the power supply.
- The ventilator should give sufficient warning to the user when the internal battery is low (e.g., by activating an audible alarm at least 20 minutes before the ventilator’s performance degrades).
Remote Alarm
- It should be possible to use a remote alarm with the ventilator.
- For use in long-term care facilities, the ventilator should communicate a device identifier, the parameter that it’s alarming for, and the alarm priority.
- We prefer that the ventilator allow an “inop” or power-loss condition to be recognized as a high-priority event, distinct from a remote alarm cable disconnection or intentional removal of the ventilator from the patient.
Test method. We examined the ventilator for the required alarm features and verified with the manufacturer that the unit can relay the specified remote alarm information.
Safety Mechanisms
Criteria
- The unit should alarm for apnea and provide backup ventilation if breathing efforts stop in a spontaneous breathing mode.
- We prefer that the ventilator relieve pressure in the breathing circuit to ambient pressure if the exhalation valve is occluded.
- If the ventilator becomes inoperative, the unit should alarm and the patient should have access to room air.
Test method. We evaluated each ventilator’s compliance with the criteria.
Human Factors Design and Safety Features
Portability
Portability is important because ventilators may be moved frequently to various places, operated on a variety of surfaces, transported in a vehicle, or carried on the back of a powered wheelchair. The ease of carrying or transporting the ventilator varies among users and situations; factors that typically contribute to a unit’s portability are considered in our criteria.
Criteria
- The unit with accessories should not be cumbersome or heavy.
- We prefer that the manufacturer have a lightweight external battery (less than six pounds) available.
- We prefer units that can operate and be easily mounted in different orientations.
- We prefer that units used for in-hospital transport have the capability to be used safely in the MR environment.
Test method. We measured each ventilator’s dimensions and weighed each unit with accessories that are necessary for transport. Throughout our testing period, we assessed each unit’s resistance to tipping over. We operated the unit while it was placed in different orientations and with the control panel alternately facing upward and to each side. We checked the ventilator’s performance at the specified settings in each orientation and noted the location of the power cord to assess whether it would interfere with positioning the ventilator.
Ease of Use
Criteria
- Ventilator operation should be self-evident to a user qualified to operate the device so that, in an emergency, it can be operated with minimal delay and risk of error.
- The primary controls, including the on/off switch and variable-setting controls, as well as breathing circuit connections and all visual displays, should be on one face of the ventilator to increase their visibility and accessibility, since the unit may be used in various orientations, especially during wheelchair transport, and all sides may not be accessible.
- The on/off switch should be protected from inadvertent shutoff.
- The controls should be easy to set, and the front-panel layout should allow the operator to locate controls and easily operate the device.
- Labels and displays should clearly and concisely identify the functions of all switches, controls, and displays. They should be easy to read in subdued light and when viewed from different angles and should be durable enough to withstand routine liquid disinfections and normal wear.
- The ventilator should have some means to prevent accidental setting changes. There should be a front-panel lock, and the unit should require that two steps be taken to change a setting (e.g., confirmation knob). Switches and other controls with rotating shafts should be mounted securely.
- The ventilator should operate without requiring the use of compressed gas.
Test method. We inspected each ventilator for compliance with these criteria.
Prevention of Misassembly
The dimensions of ventilator tubing connectors have been standardized to make different manufacturers’ equipment compatible and to reduce the incidence of disconnections and misassembly. Misassembly is a common problem that can lead to a hazardous condition, such as prevention of exhalation.
Criteria
- The possibility of misconnecting the ventilator, breathing circuit, and auxiliary equipment (e.g., air filter) should be minimized by permanent connections and/or fittings designed to prevent incorrect connection and mismatching of fittings and couplings. Where such a design is not feasible, visual indicators (e.g., labels, colors) would be beneficial to help the operator avoid connection errors.
- All fittings should resist accidental disconnection.
- It should be easy for a user to take apart and reassemble the exhalation valve for cleaning. The valve should resist incorrect reassembly.
Test method. We tested each ventilator with the manufacturer’s reusable breathing circuit, attempting to misassemble the fittings between the breathing circuit and the ventilator and to determine whether misassembly would be hazardous or difficult to detect.
To test the resistance to disconnection, we pressurized the circuit and shook the connectors for a few minutes, and observed whether fittings disconnected.
We disassembled the exhalation valve and determined whether misassembly after cleaning was likely.
Line Voltage Variation and Power Interruption
Criteria
Test method. We determined the effect of line voltage variation by operating each device at specified voltages supplied by a variable transformer. We operated the units at 95 and 135 VRMS for 30 minutes under adult ventilation conditions, tested them for performance at the specified settings, and verified that the internal charging circuit was working by measuring the voltage across the terminals of the external battery connector. We also verified that alarms for high and low pressure and for disconnection from power were functional and that each ventilator operated from its internal battery.
We operated each device on line power and used a solid-state relay to create power interruptions of 10 cycles (167 msec) and 10 seconds. We subjected the units to three interruptions at each of the specified durations, with at least 30 seconds between any two interruptions. We observed the response of the ventilators.
Quality of Construction and Ease of Servicing
Criteria
- The device should not be excessively noisy or distracting when operated in any orientation.
- The device should not have sharp edges.
- The unit should be adequately protected from fluid spills.
- The line cord connection should be secured to the ventilator, but easy to remove for replacement. There should be some means of storing the line cord when it is not in use.
- The manufacturer should provide a full range of repair and maintenance services, including service information, training courses, manuals and other service data, replacement parts, and consultation so that the ventilator can be properly cared for.
- The time between maintenance intervals must be at least six months. Intervals shorter than this may place an undue burden on the user and reduce the likelihood of compliance.
- Devices intended for use in mass-casualty care should be easy to maintain while in storage, since they may be stockpiled until needed. Required maintenance during storage should be minimal and should involve only minimal disturbance to the stored devices. We prefer that manufacturers offer a maintenance program specifically for stockpiled devices.
Test method. We evaluated the units for their construction quality and ease of servicing, considering items such as mechanical structure and the quality of materials and electrical components. We evaluated each ventilator’s noise level by assessing the pitch and intensity of the sound.
__________________________________________________________
Stockpiling Tips
Stockpiling ventilators for a mass-casualty event can be a daunting task. Organizations face a number of difficulties, including choosing a model (or combination of models) that will meet the needs of mass-casualty patients, anticipating logistical issues (e.g., finding a place where a large number of devices can be stored and easily accessed when needed), and overcoming the financial barriers to establishing an adequate-size stockpile. Although there is currently no simple stockpiling solution, we have identified some of the issues organizations need to keep in mind when evaluating their options.
Know device limitations. In our testing, we determined that battery life and oxygen consumption were the areas where all portable ventilators were most likely to have problems. In addition, the less expensive models that are being offered specifically for mass-casualty applications (which we call “supplemental mass-casualty” ventilators) have limited flow. Take these factors into account when deciding which units to purchase, and make sure that potential users are aware of any limitations that may be of concern during deployment.
Avoid AGPRs. We recommend against stockpiling automatic gas-powered resuscitators (AGPRs), another type of respiratory-support device marketed for use in mass-casualty scenarios. We don’t think those devices have the necessary features to provide the sort of respiratory support likely to be required following such a mass-casualty event. (For our full recommendations on AGPRs for mass-casualty use, see the August 2008 Health Devices.)
Remember to service stockpiled units. Keep in mind that the devices in your stockpile still need to be serviced periodically. This will probably include some testing as well as a change in batteries and possibly filters. Figuring out how to store and keep track of devices so that such maintenance can be performed quickly and easily may prove to be a challenge. We encourage facilities to work closely with the device vendor to accomplish this, as well as to educate staff on proper maintenance of stockpiled devices.
Consider a shared stockpile. One alternative to each facility maintaining a large stockpile is to develop a plan to pool resources with neighboring facilities. Organizations should also be aware of regional stockpiles that may be available to them in the event of a mass-casualty scenario. In addition, the U.S. Centers for Disease Control and Prevention operates the Strategic National Stockpile of several thousand ventilators.