This Device Evaluation webcast is being made available to the general public. Contact us to learn about everything the Device Evaluation group can do for your organization.
This webcast was held on November 16, 2022. A recording of the event appears below.
Overview
In a healthcare merger, integrating the component entities is a complex task. Not only must the organizational processes be integrated, but the medical devices and systems within those processes must often themselves be integrated. Clinical engineers play a central role in ensuring success.
During our November 16, 2022, lab webcast, presenters discussed the importance of engineering staff during a merger, addressing cybersecurity needs, and avoiding common mistakes.
Presenters
-
Jason Launders, Director of Operations, Device Evaluation, ECRI
-
Samantha Jacques, Vice President, Clinical Engineering, McLaren Healthcare
-
Erin Sparnon, Senior Engineering Manager,
Device Evaluation, ECRI
-
Juuso Leinonen, Principal Project Engineer,
Device Evaluation, ECRI
View a recording from the November 16, 2022, live-streamed lab webcast, "Mergers and Acquisitions: Assessing the Technology." Length: 0:00.
Play
Key Takeaways

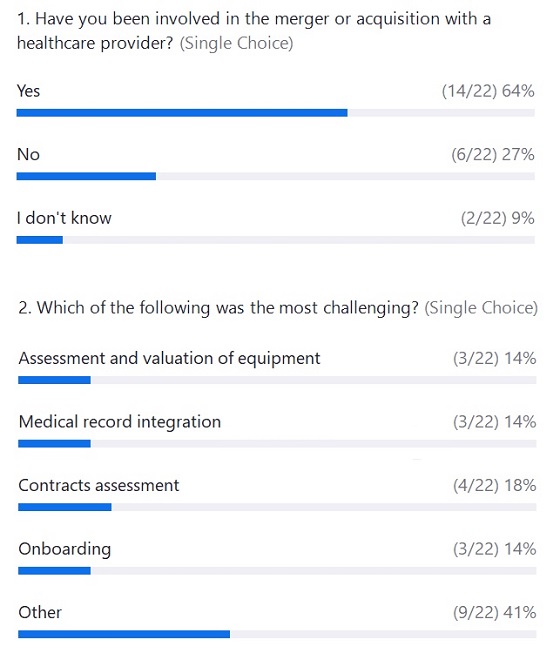
Webcast Poll Results
Presentation to AAMI Exchange
 HTM's Role in Mergers and Acquisitions
HTM's Role in Mergers and Acquisitions
View slides from Dr. Jacques' presentation to AAMI Exchange in June 2022, courtesy AAMI.
ECRI Resources
From Biomedical Guide
 Equipment Replacement Intervals
Equipment Replacement Intervals
Device Evaluation Guidance Articles
Cybersecurity Attacks Can Disrupt Healthcare Delivery, Impacting Patient Safety
Cybersecurity Risk Assessment for Medical Devices
Information Security Considerations When Decommissioning Medical Devices
Implementing the "Health Industry Cybersecurity Practices" (HICP) Guidelines
Also see: Healthcare and Public Health Sector Coordinating Council (HSCC)
Getting Started with a Cybersecurity Incident Response Plan for Your Medical Devices
Cybersecurity for Independent Healthcare Providers
Clinical Engineering and IT Collaboration on Software Maintenance
Fast Facts: How Long Do You Need to Keep Medical Equipment Records?
The Truth about Required Manufacturer Support for Discontinued Devices
2023 Health Technology Excellence Award—Submissions Now Being Accepted!
We are now accepting submissions for ECRI's annual
Health Technology Excellence Award. This award is ECRI's way of honoring a healthcare organization that has undertaken an exceptional initiative to improve patient safety, reduce costs, or otherwise facilitate better strategic management of health technology. To enter a submission, get more information, or read about the projects of previous honorees, visit the
award rules page. Don't delay—the deadline for submissions is January 31, 2023.
|