ECRI Institute's complete weekly summary of medical device hazard and recall information is available in ECRI Institute's Health Devices Alerts (HDA). For more information about HDA or other ECRI Institute services, visit
www.ecri.org or contact us at
clientservices@ecri.org.
UMDNS Device Terms
Syringes, Plunger, Enteral Feeding-Specific Connector [37614]
Tubes, Gastrostomy, Decompression/Duodenal Feeding [27144]
Tubes, Gastrostomy, Decompression/Jejunal Feeding [15998]
Tubes, Gastrostomy, Feeding [27141]
Product Identifier
[Consumable]
Enteral Devices
Geographic Regions
Worldwide
Problem
- Cross-connection of enteral tubing has led to serious consequences (e.g., feeding solution in lungs and veins).
- To help reduce the risk of cross-connections, Global Enteral Device Supplier Association (GEDSA) member manufacturers will phase out legacy (non-ENFit) devices starting July 1, 2020.
- Failure to transition could lead to delays in treatment while restocking with available products.
Manufacturer's Corrective Action/Recommendations
- FDA recommendations (letter available
here):
- Medical professionals:
- Use enteral devices that meet the ISO standards and are intended to reduce the risk of misconnection.
- Check the labeling or check with the distributor or manufacturer to determine whether your connectors meet the ISO standards.
- Organize a plan for your organization to implement the use of these new devices.
- Do not modify or adapt devices, because that may defeat their safety system.
- Minimize the use of transition adapters (a device component that forms an intermediary connection between two incompatible medical devices).
- Do not use cross-application connectors.
- Trace all lines back to their origin when reconnecting devices.
- To avoid accidental misconnections, route tubes and catheters that have different purposes in unique and standardized directions.
- GEDSA recommendations:
- Patients and caregivers:
- Use available resources to learn more about the risk of misconnections and why enteral device connecters have changed. Visit the StayConnected and
Feeding Tube Awareness Foundation websites for more information.
- Ask your retail pharmacist (i.e., community pharmacist), healthcare professional, or home medical equipment supplier about ENFit and recommendations for a successful transition.
- Patients can access more information through their respective enteral device suppliers.
- Healthcare professionals:
- Create a plan for your organization to complete a full conversion to ENFit connectors. Visit GEDSA's YouTube channel to watch the success of other organizations who have implemented ENFit.
- Educate your patients and all relevant healthcare personnel about the changes in enteral connectors.
- Communicate the ENFit conversion with the broader healthcare community in your area. E-mail
info@gedsa.org for more information about scheduling an ENFit regional summit in your area.
- Visit the StayConnected website for free resources to aid in your ENFit adoption process.
- Hospital purchasing departments and distributors:
- Identify all the enteral devices being used in your facility, determine supply needs, and develop a transition plan.
- Contact your enteral device supplier to understand the conversion process and obtain more information.
- Following the FDA's recommendations, purchase enteral devices that comply with the new ISO 80369-1 or ISO 80369-3 series standards.
- Ensure that an adequate inventory of the new devices is available to purchasers.
ECRI Recommendations
- Identify all enteral supplies including tubes, bags, administration sets, and syringes still using legacy feeding connectors.
- It is likely that these devices are from multiple suppliers.
- Conducting team meetings to ascertain all involved departments and patients is essential.
- Some tubing may remain in use for an extended period and require procedures to exchange. The next exchange should be made with consideration for ENFit compatibility. Some examples are:
- Percutaneous endoscopic gastronomy (PEG-Tube)
- Gastronomy (G-Tube)
- GastroJujenostomy (G-J Tube)
- Jujenostomy (J-Tube)
- Replace all legacy enteral feeding supplies when your current inventory is depleted.
- Adapters may be needed to connect legacy devices to newer ENFit components; however, the extra connections that are required increase the risk of misconnection and crossover.
- Adapters to connect legacy devices to ENFit will no longer be manufactured after January 2021.
Background
- FDA recognizes the hazards posed by cross-connections and has issued guidance
here.
- FDA has mandated that all new products comply with ISO 80369-3 (ENFit) or a proprietary standard incompatible with other small bore tubing.
- GEDSA estimates that hospitals in North America are about 30% converted after 2 years, based on member input and anecdotal evidence. In comparison to the European Union, this has been slower than anticipated; however, several large and prestigious hospitals or groups have converted in the summer of 2019. GEDSA expects to see an acceleration in adoption rates going into 2020 as a consequence of these large and influential institutions converting to ENFit.
- GEDSA published a plan for member suppliers to phase out legacy products.
- July 1, 2020, legacy feeding tubes and cross-application adapters will no longer be manufactured.
- January 1, 2021, transition sets and adapters sold separately from other devices will no longer be manufactured.
- Many enteral feeding sets are shipped with transition adapters.
- For related information, see ECRI Institute's 2018 Top 10 Health Technology Hazards article.
|
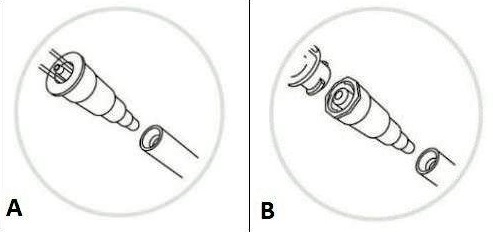 Figure 1. A. Legacy feeding tubes and cross-application adapters (manufacture end on July 1, 2020). B. Transition sets and adapters (manufacture end on January 1, 2021). Figure 1. A. Legacy feeding tubes and cross-application adapters (manufacture end on July 1, 2020). B. Transition sets and adapters (manufacture end on January 1, 2021). |
References
- United States. Food and Drug Administration. Reducing Risks through Standards Development for Medical Device Connectors [online]. 2018 Sep 6 [cited 2020 Feb 18]. Available from Internet:
Click here.
- Global Enteral Device Supplier Association (GEDSA): ENFit connector conversion letter. ENFit connector conversion schedule for the U.S. [cited 2020 Feb 20].