Numerous reports describe the risks from misconnections of enteral feeding tubing (such as tubes being connected to catheters or to non-enteral tubing) in patients across the continuum of care (hospitals, skilled nursing facilities, and at home), along with strategies to prevent them; yet these misconnections continue to occur. In response, there is a growing movement toward using a connector design commonly known by its federally registered trademarked name, ENFit.
The ENFit design uses enteral connectors whose dimensions conform to the International Organization for Standardization's ISO 80369-3 standard,[1] but it places them in a nontraditional female-to-male orientation: Administration devices have a female connector that fits around the male connector on the feeding tube (i.e., reverse of the traditional orientation).
There are many different styles, sizes, and configurations of enteral feeding devices produced by multiple manufacturers. The use of ENFit connectors will standardize the connection between all enteral devices, helping to ensure that enteral connectors will fit only with each other, and not with other connector types. A universal adapter will be needed in the short term, during the global transition to devices with ENFit connectors.
Although the ENFit initiative has received some publicity, many hospitals and clinicians that manage enterally fed patients remain unaware of or unprepared for this important initiative. In the United States, only California has mandated use of enteral feeding system devices with the new connectors.
The Problem, and Why ENFit Is a Solution
Enteral Misconnections Can Be Deadly
Misconnections of enteral feeding lines can have fatal consequences. Instances include:
- Connection of a feeding tube to a tracheostomy tube, delivering milk into an infant's lung, resulting in death
- A feeding tube connected to an in-line ventilator suction catheter, delivering feeding contents into the patient's lungs, resulting in death
- A feeding tube that was coupled with a peripheral intravenous line of a pregnant woman, resulting in enteral nutrition delivered directly into the bloodstream; neither the 35-week-old fetus nor the woman survived
Preventive Efforts
General Misconnection Prevention
ISO 80369-1:2010 specifies general requirements for small-bore connectors used in the delivery of gases and liquids for the following healthcare applications. Each delivery system class will have a distinct connector type that is physically incompatible with connectors for other delivery systems.
- Breathing systems and driving gases (80369-2)
- Enteral and gastric (80369-3)
- Urethral and urinary (80369-4)
- Limb cuff inflation (80369-5)
- Neuraxial devices including epidural and regional anesthesia devices (80369-6)
- Intravascular and hypodermic designated use of the traditional Luer connectors (80369-7)
The Joint Commission (TJC) issued a
Sentinel Event Alert on misconnections in 2014 that announced ISO's development of connector standards for these six healthcare applications, including enteral feeding. The alert instructed clinicians about misconnection prevention and presented steps for healthcare organizations to help manage risk during the transition to application-specific connectors. (TJC has not yet updated the alert to reflect completion of standards for four of those six applications.)
Enteral Misconnection Prevention
FDA collaborated with ISO to develop the ISO 80369-3 standard for enteral applications and, in February 2015, officially recognized the standard and issued specific
guidance to manufacturers encouraging adoption. The standard describes the dimensions and requirements for the design and functional performance of small-bore enteral connectors, including enteral feeding sets, enteral syringes, and patient interface devices, including access ports. ISO 80369-3 was published in July 2016.
As noted, the ENFit design takes the connectors specified in the standard and switches them, putting them in a female-to-male orientation. The Global Enteral Device Supplier Association (GEDSA), a nonprofit trade association, provides guidance documents on ENFit adoption. GEDSA's
Stay Connected website describes the new connectors and provides tools for transitioning to devices equipped with them.
The New Connectors
In an ENFit system, all administration sets (i.e., pump sets, gravity sets, bolus feeding devices) and ENFit tip syringes have a female connector that fits around the male connector on the feeding tube (i.e., nasogastric [NG] tube, gastrostomy tube [G-tube], percutaneous endoscopic gastrostomy [PEG] tube, jejunostomy tube [J-tube]). Specific enteral connector changes can be summarized as follows:
- The feeding tube change from male—the stepped or Christmas tree connector—to the new ENFit female connector. The feeding tube port for the administration set changes from female to male. (See top left image, below.)
- A temporary transition set (adapter) allows attachment to current feeding ports until new ENFit enteral feeding tubes are available. (See top right image.)
- Syringes to administer medicine or to flush, hydrate, or bolus feed through enteral tubes have an enteral-specific tip. (See bottom left image.)
- To ensure small-volume dosing accuracy, syringes ≤5 mL may be needed with an ENFit Low Dose Tip. (See bottom right image.)
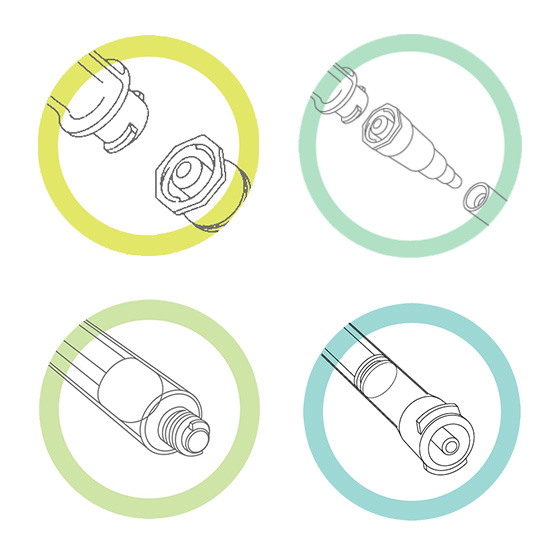 Top left: ENFit enteral connections, with new male end on feeding tube (bottom) and female end on administration set (top). ENFit has a locking feature for a more secure, leak-free connection.
Top right: Temporary adapter for connecting ENFit enteral administration sets to legacy feeding tubes and ports.
Bottom left: Enteral syringe with standard tip ENFit connection.
Bottom right: Enteral syringe with Low Dose Tip ENFit connection. (Syringe sizes of 5 mL or smaller may require one of these connectors to ensure small-volume dosing accuracy.) (Images courtesy of GEDSA.) Top left: ENFit enteral connections, with new male end on feeding tube (bottom) and female end on administration set (top). ENFit has a locking feature for a more secure, leak-free connection.
Top right: Temporary adapter for connecting ENFit enteral administration sets to legacy feeding tubes and ports.
Bottom left: Enteral syringe with standard tip ENFit connection.
Bottom right: Enteral syringe with Low Dose Tip ENFit connection. (Syringe sizes of 5 mL or smaller may require one of these connectors to ensure small-volume dosing accuracy.) (Images courtesy of GEDSA.) |
It is important to note that there is a 10-year history supporting the introduction of connectors in the "reverse," female-to-male, orientation. In 2007, the National Health System (NHS) in the United Kingdom determined that the issue of tubing misconnections must be addressed. Ahead of the ISO standards efforts, NHS and U.K. manufacturers introduced a reverse Luer system specifically designated for all enteral feeding devices. The design is very similar to and functions the same as the ENFit feeding system, though dimensionally different. However, the reverse Luer enteral feeding system was not considered to be an appropriate global solution, because of the continuing potential to connect to Luer fittings associated with other clinical applications. Nevertheless, the reverse Luer system was used for years without misconnections, supporting implementation of a reverse system like the ENFit.
Transitioning to ENFit
Healthcare providers in the United States and throughout the world should move to adopt the new ENFit connectors. Since enteral feeding patients are more mobile than ever and can travel widely between healthcare settings, the transition to ENFit system connectors is considered a global initiative. In markets like the Netherlands, Australia, and New Zealand, the transition is estimated to be more than 50% complete, and is above 80% in the United Kingdom. In many other markets, including Belgium, Germany, France, Italy, and Spain, the transition is well under way. Despite the California legislation mandating ENFit use, however, the transition in the United States and Canada has been delayed—primarily due to concerns over adequate supply of important enteral feeding system components bearing the new connectors. Repeated delays of the introduction of ENFit have in fact caused some U.S. facilities to question if the transition will ever happen. Today, however, this skepticism has been allayed by examples of facilities successfully adopting ENFit.
A successful transition will include the use of ENFit-compatible connectors on all components of an enteral feeding system. Feeding tubes and medication ports on feeding sets with new ENFit male connectors will require new female ENFit tip syringes. Syringes for flushing, hydration, bolus feeding, and enteral administration of medication are critical to support the introduction of feeding tubes with the ENFit connectors. ENFit-compatible draw-up devices such as straws or fill caps are needed for accurate dosing of small doses of meds and for filling syringes.
To ensure small-volume dosing accuracy with 5 mL and smaller syringes, the ENFit Low Dose Tip syringe may be required. Manufacturers have collaborated to validate the Low Dose Tip through independent laboratory performance testing, usability studies, and misconnection risk assessments. Testing has demonstrated that Low Dose Tip syringes can provide dosing accuracy consistent with existing male (oral) tip syringes and better than other reverse-gender solutions used today. FDA has reviewed all validation studies, and several manufacturers have gained 510(k) premarket clearance on their ENFit Low Dose Tip syringes.
Timeline for Implementation
To avoid disruption of enteral therapy, a careful and methodical transition to new connectors must occur in the United States—ideally, over the course of 2017. Manufacturers are now gearing up to provide the required supply of products with the ENFit connector design that comply with the new ISO standard. ECRI Institute strongly encourages all providers of enteral therapy to transition to ENFit supplies.
California law (AB 444), which has been in effect since July 1, 2016, requires health facilities (i.e., acute care hospitals, psychiatric facilities, skilled nursing facilities) to purchase accessories for enteral, epidural, and IV applications that are designed with connectors specific to that application. California hospitals continue to face implementation delays because of gaps in supply from manufacturers and distributors. However, several facilities in California have made full transition with minimal disruption to date.
Introduction of ENFit at your facility or health system may vary depending on supplier timing. GEDSA encourages manufacturers to introduce, and healthcare facilities to adopt, ENFit tip syringes and feeding tubes as soon as possible to meet the California mandate. Manufacturers also need to anticipate short- and long-term nationwide demand for the same products. And healthcare facilities need to work with their supplier representative and distributor networks to understand their specific plans for conversion. In particular, confirm that your syringe and feeding tube suppliers have adequate supplies of syringes with the new connectors before converting your facility to ENFit feeding system products. When the transition is complete, the need for legacy devices will be low, and in time, they will no longer be available.
It is highly recommended—by ECRI Institute, GEDSA, and others—that North America, Europe, Middle East, Africa, Australia, and New Zealand begin to adopt the ENFit connectors as soon as there are adequate supplies of enteral feeding tubes and ENFit tip syringes. This should include ENFit Low Dose Tip syringes. GEDSA anticipates that Latin America and most of Asia will begin to transition their administration sets in the second half of 2017; for China and Japan, the changes are more likely to take place in 2018. Check with your supplier representative for more precise timing in your area. And visit GEDSA's
Stay Connected website for up-to-date information on ENFit.
Implementation Challenges
ECRI Institute has consulted with a large health system planning to adopt ENFit technology and also some early adopters. They describe the implementation process as "labor intensive and confusing," citing the following challenges:
- Communication with vendors; not all sales representatives are familiar with ENFit
- Knowledge gaps on the part of key healthcare facility stakeholders
- Obtaining manufacturer reference numbers for all ENFit products within the supply chain
- Availability of enteral supplies with ENFit connectors
- Clinician training
- Implementation delays and timelines
- Temporary adapter costs
- Stocking and managing inventory in medication and supply rooms
- Communications between prescribers and pharmacists regarding route of medication administration
- Competing planning and time commitments required for adoption of the ISO 80369-6 small-bore connector initiative for neuraxial (epidural and spinal) connectors (named NRFit)
One large syringe supplier (BD Medical), which was involved during the initial start of the standard harmonization and was once a member of GEDSA, has indicated it no longer supports the ISO 80369-3 connector design for use in a female-to-male ENFit orientation, citing concerns about dose accuracy (which GEDSA disagrees with). However, many other suppliers are working to provide adequate supplies of ENFit tip syringes to meet market demand.
Recommendations
- Assemble an interdisciplinary team (e.g., dietitian, risk manager, pharmacist, nursing, supply chain representative, and home and postacute care representatives)
- Engage discharge planners and their respective home care providers who manage postdischarge supply needs for patients at home and in skilled nursing facilities
- Determine a transition plan and timing
- Communicate your go-live plans with facility/system staff to make them aware that the transition is in a planning phase
- Plan to train personnel on transitioning to ENFit feeding devices
- Partner with suppliers (manufacturers and distributors)
- Understand what products from each of your enteral device suppliers are or will become available
- Forecast facility/system demand for ENFit feeding devices and transition adapters
- Identify a second and third supplier of syringes to ensure adequate ongoing supply
- Develop a crosswalk of all legacy and corresponding ENFit devices with their new item numbers from each supplier
- Have a contingency plan for what to do when a product is not available
- Make sure to communicate your go-live date
- Take advantage of information and tools from GEDSA
- Sign up for GEDSA newsletters to stay on top of news and updates
- Utilize resources of the
Stay Connected website to plan your transition
_____________________________________________________
[1] ISO 80369-3:2016, Small-Bore Connectors for Liquids and Gases in Healthcare Applications—Part 3: Connectors for Enteral Applications. Available for purchase from ISO:
https://www.iso.org/standard/50731.html.