ECRI's 14th Health Devices Achievement Award is shared by two organizations from Vancouver, British Columbia, Canada: Lower Mainland Biomedical Engineering, which is part of the Provincial Health Services Authority, and Vancouver Coastal Health. The organizations were honored for their joint effort to identify the root cause of overinfusions involving a commonly used brand of infusion pump. The investigative efforts and persistence of the team members improved patient safety not only within their area of responsibility (British Columbia), but throughout the world.
The Health Devices Achievement Award recognizes outstanding initiatives undertaken by member healthcare institutions to improve patient safety, reduce costs, or otherwise facilitate better strategic management of health technology. ECRI announced the winner of the 14th award in November 2020. For details about the other submissions that achieved recognition, see
The Health Devices Achievement Award: Recognizing Exceptional Health Technology Management.
ECRI congratulates all the project team members, in particular Brendan Gribbons, Carol Park, Sarah Hawley, Brittany Watson, Emily Rose, Allison Muniak, Darren Kopetsky, and Steven Gorelik.
 Vancouver General Hospital. (Image courtesy of Vancouver Coastal Health.)
Vancouver General Hospital. (Image courtesy of Vancouver Coastal Health.) |
The Challenge
To identify the root cause for the high incidence of overinfusions across British Columbia involving a commonly used brand of infusion pump.
The Context
In July 2018 in a British Columbia hospital, a patient received an infusion of 100 mg of IV morphine in less than 15 minutes that was intended to be administered over a period of several hours—an event that had the potential to be fatal. The infusion pump that was used to administer the medication had been set up properly and was programmed to deliver the dose at the proper rate; yet the overinfusion occurred.
Following this incident, 33 more confirmed IV overinfusion events involving this same model of pump were identified in healthcare facilities across British Columbia. In most of these cases, staff had been unable to pinpoint a clear cause of the failure.
The high risk to patients, the wide dispersion of this pump model (approximately 12,000 of the pumps had been in use across the province), and a building distrust of the pump among clinicians made identifying the underlying problem critically important.
The Process
The overinfusion incidents spurred a systematic 10-month, multidisciplinary investigation led by Lower Mainland Biomedical Engineering (LMBME)—part of the Provincial Health Services Authority in British Columbia—and Vancouver Coastal Health (VCH). LMBME is a health technology management organization that supports VCH, Fraser Health Authority, and Providence Health Care in British Columbia. The investigation team included LMBME staff; several departments within VCH, including Quality and Patient Safety, Nursing Professional Practice, and Risk Management; and point-of-care clinicians, senior leadership, and the pump's manufacturer. ECRI was also called in at one point to help with aspects of the investigation.
Improving Evidence Collection
One early roadblock to the investigation was that the evidence received from most of the initial incidents was poor: Infusion tubing sets had been discarded, devices had been returned to service, and the infusion system had often been modified from its "as-is" state at the time of the overinfusion.
To improve the quality of information available for analysis when an incident occurred, frontline staff were provided with resources and education on the steps to take to sequester the infusion system when an overinfusion was suspected. Staff were advised to secure the entire circuit, preserve evidence to aid in the investigation, and document the event in the province's Patient Safety and Learning System.
Additionally, LMBME and the infusion pump manufacturer collaborated to develop an overinfusion investigation procedure. This procedure defined specific steps for LMBME to take during the pump recovery and investigation process to maximize evidence collection and reduce evidence alteration that might impact the manufacturer's subsequent investigation.
In parallel, a communication strategy was implemented to raise awareness about the potential for overinfusion and to provide tips on enhanced vigilance when infusing medication. The team developed communication tools that would optimize the spread of the message while not raising alarm among the thousands of staff whose participation and vigilance were critical to the investigation.
Digging Deeper
The manufacturer initially focused on user education to reinforce proper set-loading procedures, stating that this strategy had been successful in decreasing overinfusion incidents at other hospitals. But the investigation team was skeptical that user error was the root cause of the incidents it was seeing. User education was nevertheless provided—it did not lead to any observable decrease in the incidence of overinfusions—while the team persisted in the investigation.
Catching a Break
New evidence received from overinfusions occurring in March 2019 significantly changed the course of the investigation. A nurse identified a pump that continued to flow while in the pause mode; the nurse video-recorded the unintentional medication flow and immediately engaged the investigation team. Another nurse observed unrestricted flow from a pump that was turned off and secured the entire circuit. With the excellent information and material from these incidents in hand, the investigation team, the infusion pump manufacturer, and ECRI collaboratively interrogated the infusion system.
The Results
The collaborative interrogation of the system revealed that the tubing was a factor contributing to the uncontrolled flow. VCH and LMBME further examined the tubing by scanning it with a micro-CT scanner. The scans revealed that the tubing was not concentric—that is, the tubing wall was thicker on one side of the lumen than the other (see Figure 1).
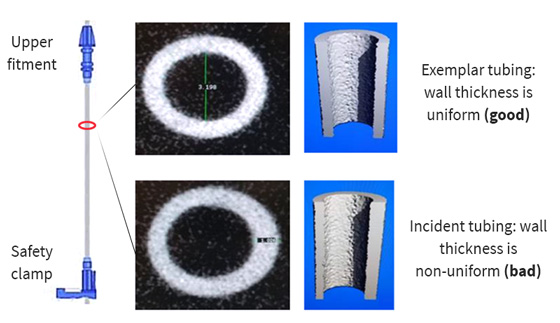 Figure 1. Scans of infusion tubing showing a tubing set with uniform wall thickness (top) and one with the tubing wall thicker on one side than the other (bottom).
Figure 1. Scans of infusion tubing showing a tubing set with uniform wall thickness (top) and one with the tubing wall thicker on one side than the other (bottom).
|
The consequence was that when the tubing was oriented a specific way within the pump, the increased wall thickness on one side could prevent the pump from fully occluding the tubing, thus allowing flow. When the tubing was oriented in other ways, however, the pump could fully occlude the tubing, as intended (see Figure 2).
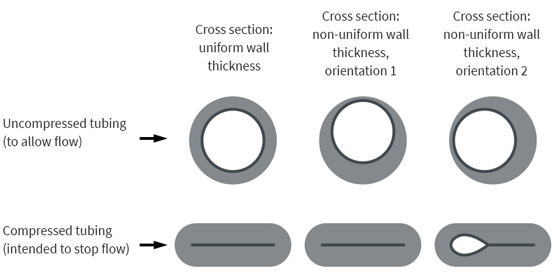 Figure 2. Cross sections of infusion tubing shown when uncompressed to allow flow (top row) and after being compressed by the pump to stop the flow (bottom row). The images on the left show tubing with uniform wall thickness. The images in the center and on the right show tubing with non-uniform wall thicknesses; when such tubing is oriented in a certain way within the pump (right-hand images), the pump cannot fully compress the tubing, allowing unintended flow through the tubing.
Figure 2. Cross sections of infusion tubing shown when uncompressed to allow flow (top row) and after being compressed by the pump to stop the flow (bottom row). The images on the left show tubing with uniform wall thickness. The images in the center and on the right show tubing with non-uniform wall thicknesses; when such tubing is oriented in a certain way within the pump (right-hand images), the pump cannot fully compress the tubing, allowing unintended flow through the tubing. |
The discovery led the pump manufacturer to examine its inventory and quality control systems. The manufacturer worked with suppliers to identify the root cause of the tubing abnormality, and took steps to remove affected lots from service:
1. The company initially identified and recalled 75 lots that potentially contained the abnormal tubing and that had been sent to British Columbia.
2. In the summer of 2019, the recall was expanded globally, affecting hundreds of millions of tubing sets. (In the United States alone, over 150 million tubing sets were recalled.)
Internally, the investigation prompted LMBME and VCH to improve their processes for identifying when medical device incidents occur and for investigating incidents in a systematic and comprehensive manner. Steps include:
1. Ensuring that medical device incidents are reported to LMBME and are entered in British Columbia's Provincial Patient Safety and Learning System
2. Implementing a systematic process for responding to medical device incidents and for sequestering devices
3. Developing teaching resources, infographics, and lanyard cards that remind staff about the incident-reporting process
Key Takeaways
The organizations' efforts improved patient safety not only within their area of responsibility (British Columbia), but throughout the world.
1. The tubing's dimensional defect, first identified by the LMBME/VCH investigation team, quite possibly explains years of unexplained overdeliveries of medications with this brand of infusion pump.
2. ECRI published several
Health Devices Alerts articles related to the tubing issues and overinfusion risks uncovered by this investigation. See, for example:
a)
BD—Alaris Pump Modules: Overinfusion May Occur with the Use of Certain Administration Sets [ECRI Exclusive Hazard Report]. Published 2019 Apr 30; updated 2019 May 2 (Accession No. H0511).
b)
BD—Alaris Pump Administration Sets: Defect May Cause Unintended Delivery of Medication (Canada) [Update]. Published 2019 Apr 26; updated 2019 May 15 (Accession No. A32600 02).
Following are a few of the lessons learned and key points illustrated by this investigative effort:
1. Setting the proper tone can pay dividends. The proactive and supportive approach that the multidisciplinary investigation team used during a time of uncertainty and ambiguity kept staff informed and set a tone for a culture of learning and sharing.
2. Direct care providers play a critical role by remaining vigilant and by reporting incidents and preserving evidence when an event occurs. Medical device manufacturers, regulatory authorities, investigative agencies, and other hospitals can't respond to problems—or understand the full breadth of problems—if incidents are not reported whenever they occur and if available evidence is not preserved.
3. Multidisciplinary investigation teams are highly effective. The tubing dimensional defect was exceptionally difficult to detect. Every stakeholder—from the frontline nurse to biomedical engineering—played a role in the processes that led to identifying the tubing defect.
4. Conclusions should be driven by the evidence, not based on preconceptions. The manufacturer posited that improved user education could address the issues being observed, since that approach had been effective in other locations. But the investigation team did not agree that the evidence supported this conclusion and continued to dig into the issue. Their persistence led to the discovery of the tubing defect.
5. The inability of infusion pumps to measure flow through the tubing remains a key limitation of the current technology. All infusion pump models can fail in such a way that the actual flow rate exceeds the programmed flow rate.