John Muir Health (Walnut Creek, CA) has been named a finalist for ECRI Institute's 13th Health Devices Achievement Award. The organization was one of two groups honored for detailing its implementation of an alternative equipment maintenance (AEM) program—an approach for completing preventive maintenance (PM) activities in a more effective and efficient manner. (The other was the
VA New England Healthcare System.)
The Health Devices Achievement Award recognizes outstanding initiatives undertaken by member healthcare institutions to improve patient safety, reduce costs, or otherwise facilitate better strategic management of health technology. For details about the winning submission and other finalists, see
The Health Devices Achievement Award: Recognizing Exceptional Health Technology Management.
ECRI Institute congratulates Nader Hammoud, Cindy Pocklington, and the rest of the John Muir Health team.
The Challenge
To establish a user-friendly, efficient system for implementing an AEM program that complies with Joint Commission PM requirements.
The Context
As noted by the U.S. Centers for Medicare & Medicaid Services (CMS), an AEM program allows a healthcare facility to "adjust its maintenance, inspection, and testing frequency and activities for facility and medical equipment from what is recommended by the manufacturer, based on a risk-based assessment by qualified personnel."[1] Such programs can be an acceptable approach for completing PM activities in a more effective and efficient manner, provided that certain conditions are met. As such, AEM programs allow clinical/biomedical engineering departments to direct time and resources toward other activities that have a greater impact on patient safety. For additional information, see
What Is an Alternative Equipment Maintenance Program?
Ever since AEM programs were introduced as an acceptable alternative to rigid adherence to manufacturer-recommended maintenance activities, the team at John Muir Health had recognized the need to implement such a program. Like most clinical/biomedical engineering departments, the group was always on the lookout for ways to reduce avoidable expenditures of time and other resources so that efforts could be directed to more impactful patient safety activities.
However, the idea of formalizing a plan that specifies deviating from manufacturer-recommended maintenance activities was approached with some trepidation. The organization was wary of introducing practices that might be deemed to be noncompliant with Joint Commission requirements. Thus, implementing an AEM program would require learning the ins and outs of those requirements and instituting a well-reasoned process that satisfied stakeholders' concerns.
The Process
The first step involved education. Department leadership attended educational sessions and used other industry resources to better understand what needed to be done, how and when to do it, and who to involve in the process. Doing so allowed the group to move the AEM idea from the "taboo" list to the "possible" list.
Next was defining a process for determining which equipment could qualify for AEM status, how those decisions would be documented, and what information needed to be accessible to management and others to show compliance. The department manager decided to create a tool—a database developed in Microsoft Access—that could accomplish the needed tasks.
Key aspects of the database include the following:
1. The database helps assess and document a device's appropriateness for AEM status, using logic programmed into the system and user-entered data for the device in question. An example database entry for physiologic monitors is presented in the screenshot below. Note the following steps that can be seen in the figure:
a) The user enters a determination of the Severity Level for the device in question, referencing the criteria outlined on the screen.
b) The database calculates a Probability Score based on maintenance data that the user enters. The user enters the number of work orders, the number of devices in inventory, the number of PM-preventable failures that the facility has experienced, and the length of time covered by the data. The database uses this information to calculate the "mean time between failures" (MTBF), a concept that can be used to assess how often a device is expected to fail. The Probability Score is based on the MTBF value.
c) The database calculates a risk score (which is defined in the grid at the top-right of the screen). This score contributes to the AEM assessment.
2. Documentation of the decision-making process for any device can be easily retrieved from the database (e.g., for surveyors or an audit).
3. The database also calculates an estimated time saving associated with eliminating PM tasks for equipment that qualifies for AEM status based on historical PM data.
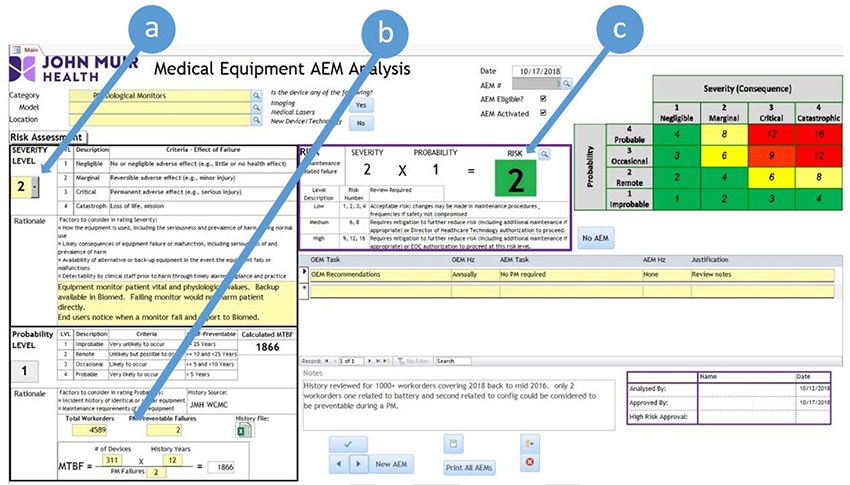
(Click to enlarge image)
An example entry from John Muir Health's Medical Equipment AEM Analysis database. Note the following steps that can be seen in this entry for physiologic monitors: (a) The user enters a Severity Level. (b) The user enters relevant maintenance data. (c) The database calculates the risk level, which contributes to the AEM determination. (Figure courtesy of John Muir Health.) |
The Results
The end result is a well-designed Access database that requires minimal user involvement:
1. Users enter information about a device into a few basic fields.
2. The database then calculates the risk level associated with that equipment and whether the device can be accepted as appropriate for AEM.
3. The database can also be queried to retrieve AEM profiles based on the content of any field (e.g., model, risk level, location) or to produce documentation of all actions taken.
Thus far, John Muir Health has used the database to identify more than 10 AEM-eligible device types, covering about 1,000 individual devices. The database helps in tracking total time saved.
Key Takeaways
By building the decision logic into the system, the database both formalizes and simplifies the AEM decision-making process. Users need to enter only minimal information; the database does the rest.
Additionally, the database's documentation capabilities simplify the record-keeping function. Users can retrieve information about the AEM decision for any device on demand, whether for monthly reports, to demonstrate time savings, or in response to an audit.
___________________________________
[1] Hospital equipment maintenance requirements [Survey & Certification Letter]. S&C:14-07-Hospital. 2013 Dec 20. Available from:
https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-14-07.pdf.